What Is The Particular Significance Of Valence Electrons
Holbox
Mar 19, 2025 · 7 min read
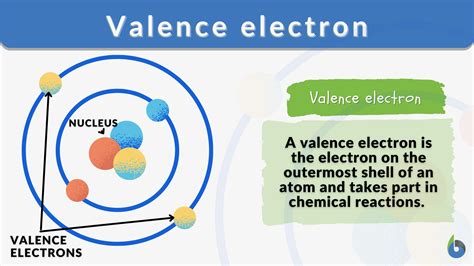
Table of Contents
What is the Particular Significance of Valence Electrons?
Valence electrons are the cornerstone of chemistry, governing how atoms interact to form molecules and influencing the properties of matter. Understanding their significance unlocks a deeper comprehension of the world around us, from the formation of simple water molecules to the complexities of advanced materials. This article delves into the crucial role valence electrons play, exploring their influence on chemical bonding, reactivity, and the periodic trends observed across the elements.
Defining Valence Electrons: The Outermost Players
Valence electrons are the electrons located in the outermost shell, or energy level, of an atom. These electrons are the furthest from the atom's nucleus and experience the weakest electrostatic attraction to the positively charged protons within the nucleus. This weaker attraction is key to their high reactivity. They are the primary participants in chemical reactions, determining an atom's bonding capacity and chemical behavior. Think of them as the "ambassadors" of the atom, interacting with other atoms to establish connections.
Unlike core electrons, which are tightly bound to the nucleus and largely uninvolved in chemical reactions, valence electrons are relatively free to move and interact with electrons from other atoms. Their involvement in chemical bonding allows atoms to achieve a more stable electron configuration, typically resembling the stable electron configuration of a noble gas. This drive towards stability is the driving force behind many chemical reactions.
Identifying Valence Electrons: A Quick Guide
Determining the number of valence electrons for an element is relatively straightforward. The easiest method involves using the element's group number (vertical column) on the periodic table. For Groups 1A through 8A (the main group elements), the group number directly corresponds to the number of valence electrons. For example:
- Group 1A (Alkali Metals): 1 valence electron (e.g., Lithium (Li) has one valence electron)
- Group 2A (Alkaline Earth Metals): 2 valence electrons (e.g., Magnesium (Mg) has two valence electrons)
- Group 3A: 3 valence electrons
- Group 4A: 4 valence electrons
- Group 5A: 5 valence electrons
- Group 6A (Chalcogens): 6 valence electrons
- Group 7A (Halogens): 7 valence electrons
- Group 8A (Noble Gases): 8 valence electrons (except Helium, which has 2)
Transition metals (in the d-block) have more complex valence electron configurations, with valence electrons occupying both the outermost s and d subshells. This contributes to their ability to exhibit multiple oxidation states.
The Significance of Valence Electrons in Chemical Bonding
The primary significance of valence electrons lies in their role in chemical bonding. Atoms strive for stability, usually achieved by acquiring a full outer electron shell, a configuration similar to that of noble gases (octet rule). This drive toward stability dictates how atoms interact and form chemical bonds. There are three primary types of chemical bonds:
1. Ionic Bonds: Electron Transfer for Stability
Ionic bonds form through the complete transfer of one or more valence electrons from one atom to another. This transfer creates ions: positively charged cations (atoms that have lost electrons) and negatively charged anions (atoms that have gained electrons). The electrostatic attraction between these oppositely charged ions constitutes the ionic bond. A classic example is the formation of sodium chloride (NaCl), where sodium (Na) loses one valence electron to chlorine (Cl), forming Na⁺ and Cl⁻ ions that are held together by strong electrostatic forces.
The number of valence electrons directly influences the charge of the resulting ions. Atoms tend to lose or gain electrons to achieve a stable octet (or duet for hydrogen and helium), resulting in ions with specific charges.
2. Covalent Bonds: Shared Electrons, Shared Stability
Covalent bonds arise when atoms share valence electrons to achieve a stable electron configuration. This sharing creates a strong bond between the atoms, holding them together in a molecule. Unlike ionic bonds, there is no complete transfer of electrons; instead, the shared electrons are attracted to the nuclei of both atoms, forming a region of high electron density between them. Examples include the bonds in water (H₂O), methane (CH₄), and countless other organic molecules.
The number of valence electrons determines the number of covalent bonds an atom can form. For instance, carbon (with four valence electrons) typically forms four covalent bonds, while oxygen (with six valence electrons) usually forms two.
3. Metallic Bonds: A Sea of Electrons
Metallic bonds occur in metals, where valence electrons are delocalized, forming a "sea" of electrons that are shared among a lattice of metal cations. This mobile sea of electrons accounts for many characteristic properties of metals, such as high electrical and thermal conductivity, malleability, and ductility. The number of valence electrons contributes to the strength of the metallic bond and the resulting properties of the metal.
Valence Electrons and Chemical Reactivity
The reactivity of an element is directly linked to its number of valence electrons. Atoms with nearly full or nearly empty valence shells are highly reactive, as they readily gain or lose electrons to achieve stability. Conversely, atoms with half-filled or fully filled valence shells (like noble gases) are relatively unreactive.
- Highly Reactive Elements: Alkali metals (Group 1A) and halogens (Group 7A) are highly reactive due to their single valence electron (easily lost) and seven valence electrons (easily gained), respectively.
- Less Reactive Elements: Elements with four valence electrons (Group 4A) can exhibit varying reactivity, depending on whether they tend to lose or share electrons.
- Inert Elements: Noble gases (Group 8A) have a full valence shell (octet), making them exceptionally unreactive.
This reactivity dictates how elements interact with each other, forming various compounds and materials with vastly different properties.
Valence Electrons and Periodic Trends
The periodic table's arrangement reflects the systematic variation in the number of valence electrons across elements. This systematic variation leads to observable periodic trends in properties like ionization energy, electronegativity, and atomic radius.
-
Ionization Energy: The energy required to remove a valence electron from an atom. Ionization energy generally increases across a period (left to right) as the nuclear charge increases, holding the valence electrons more tightly. It generally decreases down a group (top to bottom) as the valence electrons are farther from the nucleus.
-
Electronegativity: The ability of an atom to attract electrons in a chemical bond. Electronegativity generally increases across a period and decreases down a group, mirroring the trends in ionization energy.
-
Atomic Radius: The size of an atom. Atomic radius generally decreases across a period and increases down a group. The increase down a group reflects the addition of electron shells.
These periodic trends are directly influenced by the number and arrangement of valence electrons, demonstrating the fundamental role valence electrons play in determining the macroscopic properties of elements and their compounds.
Valence Electrons and Advanced Materials Science
The understanding of valence electrons is not confined to basic chemistry; it extends to the cutting edge of materials science. The design and synthesis of novel materials with specific properties often involve manipulating the number and interactions of valence electrons. Examples include:
-
Semiconductors: Semiconductors, like silicon and germanium, have valence electron configurations that allow for controlled electrical conductivity, crucial for electronics. Doping semiconductors with impurities alters their valence electron configuration, fine-tuning their electrical properties.
-
Superconductors: The quest for superconductors with zero electrical resistance at high temperatures involves understanding how valence electrons interact to create these remarkable properties.
-
Nanomaterials: Manipulating the valence electrons in nanomaterials allows for tailoring their optical, magnetic, and catalytic properties. The arrangement and interaction of valence electrons at the nanoscale significantly influence the material's behavior.
Conclusion: The Universal Influence of Valence Electrons
In conclusion, valence electrons hold paramount significance in chemistry and materials science. Their role in chemical bonding, reactivity, and periodic trends is undeniable. Understanding valence electron configurations provides a framework for predicting chemical behavior, designing novel materials, and interpreting the properties of the diverse substances that make up our world. From the simplest molecules to the most advanced technologies, the significance of valence electrons remains central to our understanding of the material world. Their influence permeates every aspect of chemical interactions, making them a cornerstone concept for anyone seeking a deeper understanding of chemistry and the physical sciences. The continued exploration and manipulation of valence electrons will undoubtedly drive future advancements in materials science and technology.
Latest Posts
Latest Posts
-
Draw The Correct Product For The Diels Alder Reaction
Mar 19, 2025
-
Which Particles Do Not Affect The Stability Of The Atom
Mar 19, 2025
-
Cash Flows From Investing Do Not Include Cash Flows From
Mar 19, 2025
-
An Indicator Of An Expanding Intracranial Hematoma
Mar 19, 2025
-
Spotlight Figure 10 10 Neuromuscular Junction Nmj
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about What Is The Particular Significance Of Valence Electrons . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
