Draw The Correct Product For The Diels-alder Reaction
Holbox
Mar 17, 2025 · 5 min read
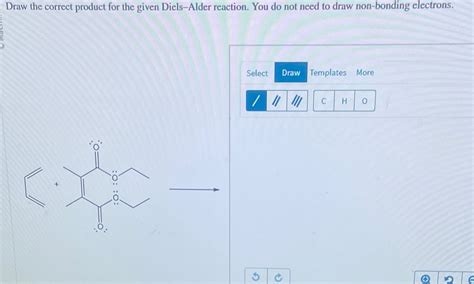
Table of Contents
Drawing the Correct Product for the Diels-Alder Reaction: A Comprehensive Guide
The Diels-Alder reaction, a cornerstone of organic chemistry, is a powerful tool for constructing six-membered rings. Understanding how to predict and draw the correct product is crucial for success in organic synthesis. This comprehensive guide will walk you through the process, covering reaction mechanisms, stereochemistry, and regioselectivity, equipping you with the knowledge to confidently predict Diels-Alder products.
Understanding the Diels-Alder Reaction: A Cycloaddition
The Diels-Alder reaction is a [4+2] cycloaddition, meaning a four-carbon π-system (the diene) reacts with a two-carbon π-system (the dienophile) to form a six-membered ring. This reaction is concerted, meaning it occurs in a single step without any intermediates. This concerted nature has significant implications for the stereochemistry of the product.
The Dienophile and the Diene: Key Players
-
The Diene: The diene must be able to adopt an s-cis conformation, meaning the two double bonds are on the same side of the molecule. This conformation allows the reaction to proceed. Steric hindrance can influence the ability of a diene to adopt the s-cis conformation.
-
The Dienophile: The dienophile can be an alkene or alkyne. Electron-withdrawing groups on the dienophile increase its reactivity by lowering its LUMO energy, making it a better acceptor of electrons. Examples of electron-withdrawing groups include carbonyl groups (C=O), nitriles (CN), and nitro groups (NO2).
Predicting the Stereochemistry of the Product
One of the most important aspects of the Diels-Alder reaction is its stereospecificity. The stereochemistry of the reactants is preserved in the product. This means:
-
Cis dienophiles yield cis products: If the substituents on the dienophile are on the same side (cis), they will remain on the same side in the product.
-
Trans dienophiles yield trans products: Similarly, if the substituents on the dienophile are on opposite sides (trans), they will remain on opposite sides in the product.
-
Endo vs. Exo Products: When cyclic dienophiles are involved, the reaction can lead to endo or exo products. The endo product is favored due to secondary orbital interactions between the diene and the dienophile. The endo rule states that the larger substituents on the dienophile will end up cis to the bridging bond in the cyclohexene ring. This is often depicted using a boat-like conformation.
Example: The Diels-Alder reaction of cyclopentadiene with maleic anhydride (a cis dienophile) yields the endo product, predominantly, due to secondary orbital interactions.
Predicting Regioselectivity: Where Do the Substituents Go?
Regioselectivity refers to the orientation of substituents in the product. In Diels-Alder reactions with substituted dienes and dienophiles, predicting the regiochemistry requires understanding the electronic effects of substituents.
-
Electron-donating groups (EDG) on the diene: EDGs on the diene direct the reaction to place the electron-withdrawing group (EWG) on the dienophile at the 1-position.
-
Electron-withdrawing groups (EWG) on the diene: EWGs on the diene direct the reaction to place the electron-withdrawing group on the dienophile at the 4-position.
-
Electron-withdrawing groups on the dienophile: EWGs on the dienophile increase the reaction rate and preferentially react at the position that allows for the greatest stabilization of the transition state through secondary orbital interactions.
Example: Consider the reaction between 1-methoxy-1,3-butadiene (an EDG on the diene) and acrolein (an EWG on the dienophile). The major product will have the aldehyde group at the 1-position of the cyclohexene ring.
Drawing the Product: A Step-by-Step Approach
Let's solidify our understanding by walking through the process of drawing the product of a Diels-Alder reaction step-by-step:
-
Identify the diene and dienophile: Clearly identify the four-carbon π-system (diene) and the two-carbon π-system (dienophile).
-
Determine the s-cis conformation of the diene: Ensure the diene can adopt the s-cis conformation. If steric hindrance is significant, the reaction may be slow or not occur at all.
-
Orient the reactants for the cycloaddition: Arrange the diene and dienophile so they can approach each other in a way that will allow the formation of the six-membered ring.
-
Form the six-membered ring: Connect the carbons of the diene and dienophile to form the cyclohexene ring. Remember that the reaction is concerted, meaning this happens in one step.
-
Add substituents, respecting stereochemistry: Add the substituents from the diene and dienophile to the newly formed cyclohexene ring. Carefully preserve the stereochemistry (cis/trans) from the reactants. Pay attention to the endo/exo selectivity if applicable.
-
Consider regioselectivity: If the diene or dienophile is substituted, carefully consider the electronic effects of the substituents to predict the regioselectivity of the product.
Advanced Considerations: Inverse Electron Demand Diels-Alder Reactions
In typical Diels-Alder reactions, the diene acts as a nucleophile (electron-rich) and the dienophile acts as an electrophile (electron-poor). However, inverse electron demand Diels-Alder reactions utilize electron-poor dienes and electron-rich dienophiles. This reversal of electronic demand changes the regioselectivity and reactivity.
Practical Applications and Significance
The Diels-Alder reaction has widespread applications in organic synthesis and is used to create various cyclic compounds with high stereoselectivity. This reaction is crucial in building complex molecules for pharmaceutical, material science, and polymer chemistry. It plays a significant role in natural product synthesis, allowing chemists to create intricate structures efficiently and predictably.
Conclusion: Mastering the Diels-Alder Reaction
Drawing the correct product for the Diels-Alder reaction requires a thorough understanding of reaction mechanisms, stereochemistry, and regioselectivity. By following the step-by-step approach outlined in this guide and considering the electronic effects of substituents, you can confidently predict and draw the products of this powerful reaction. Practice is key to mastering this crucial transformation in organic chemistry. The more you practice drawing these products, the more intuitive the process will become, leading to a deeper understanding of reaction mechanisms and structural prediction. Remember to always consider the s-cis conformation of the diene, the stereochemistry of the dienophile, and the potential influence of electronic effects to draw the most accurate representation of the Diels-Alder product. This detailed understanding forms the basis for further exploration of more complex organic reactions and synthesis strategies.
Latest Posts
Latest Posts
-
How To Cite Surveys In Mla
Mar 17, 2025
-
Wordpress Is Popular Free And Open Source
Mar 17, 2025
-
The Difference Between Aerobic And Anaerobic Glucose Breakdown Is
Mar 17, 2025
-
On December 29 2020 Patel Products
Mar 17, 2025
-
A Customer Tells His Current Sales Rep
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Draw The Correct Product For The Diels-alder Reaction . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
