Which Particles Do Not Affect The Stability Of The Atom
Holbox
Mar 19, 2025 · 5 min read
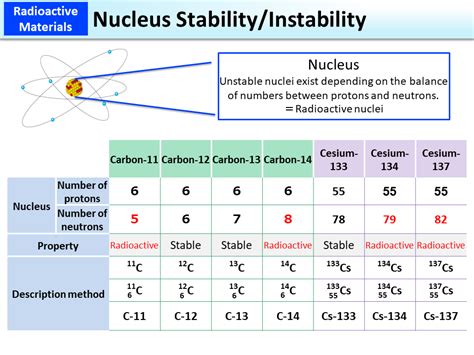
Table of Contents
Which Particles Don't Affect the Stability of an Atom? Understanding Atomic Structure and Nuclear Forces
The stability of an atom, its resistance to radioactive decay or spontaneous transformation, is a fundamental concept in physics and chemistry. It's determined by a complex interplay of forces within the atom's nucleus and the arrangement of its electrons. While many factors contribute to stability, some particles play a far more crucial role than others. This article delves into the intricacies of atomic structure, exploring which particles are essential for stability and which have a negligible impact. We'll uncover the key players and the forces that govern atomic stability.
The Nucleus: The Heart of Atomic Stability
The atom's nucleus, a tiny, dense core, houses the majority of its mass. It's composed primarily of two types of particles: protons and neutrons. These are collectively known as nucleons.
Protons: Positive Charge and Identity
Protons carry a positive electrical charge, and their number defines the atomic number (Z) of an element. This determines the element's chemical properties and its place on the periodic table. The number of protons is crucial for atomic identity and indirectly influences stability. A change in the number of protons transforms the atom into a different element. While the number of protons is key, their individual characteristics don't directly affect stability in a way that individual neutrons or electrons do.
Neutrons: Neutral Charge and Stability Influence
Neutrons, as their name suggests, carry no electrical charge. Their presence in the nucleus is pivotal for nuclear stability. The neutron-to-proton ratio is a critical factor. Too few neutrons relative to protons can lead to instability, resulting in radioactive decay. The strong nuclear force, which overcomes the electrostatic repulsion between positively charged protons, is significantly influenced by the number of neutrons. An optimal neutron-to-proton ratio helps to strengthen this force, thus enhancing stability. However, it's the number of neutrons that affects stability, not the individual properties of each neutron itself.
Electrons: Orbiting the Nucleus, Peripheral Influence
Electrons are negatively charged particles that orbit the nucleus in shells or energy levels. Their number equals the number of protons in a neutral atom. While electron configuration significantly influences an atom's chemical behavior, its reactivity, and its ability to form chemical bonds, electrons have a minimal direct impact on the nucleus's inherent stability.
Electron Configuration and Chemical Stability, Not Nuclear Stability
The arrangement of electrons in energy levels, often depicted using electron shell diagrams, determines an atom's chemical stability. Atoms strive to achieve a stable electron configuration, often a full outermost shell (octet rule), which dictates their chemical reactivity. However, this chemical stability is distinct from nuclear stability. Nuclear stability involves the forces within the nucleus itself, and electrons are too far removed to directly influence these forces. Their interactions are primarily electromagnetic, weak compared to the strong nuclear force governing nuclear stability.
Other Particles: Minimal to No Impact on Atomic Stability
Beyond protons, neutrons, and electrons, other particles exist, but their influence on an atom's stability is negligible. These include:
Positrons: Antimatter Counterparts
Positrons are the antimatter counterparts of electrons, possessing the same mass but a positive charge. Their interaction with electrons leads to annihilation, converting their mass into energy. However, positrons are not typically found within stable atoms; they are produced in certain radioactive decay processes. Therefore, they don't contribute to the baseline stability of a typical atom.
Neutrinos: Elusive Particles
Neutrinos are incredibly light, neutral particles that interact very weakly with matter. They are involved in certain types of radioactive decay, carrying away energy and momentum. However, their impact on an atom's overall stability is minuscule.
Other Subatomic Particles: Beyond the Standard Model
Physics delves into a vast array of subatomic particles beyond the standard model of particle physics (quarks, gluons, etc.). While these particles contribute to the fundamental structure of matter, their direct role in determining the stability of a typical atom is extremely limited and can be considered negligible for practical purposes.
The Strong Nuclear Force: The Glue Holding the Nucleus Together
The stability of an atom hinges on the strong nuclear force, a fundamental force far stronger than the electromagnetic force. It's responsible for binding protons and neutrons together in the nucleus, counteracting the electrostatic repulsion between protons.
The Role of Neutrons in Strengthening the Strong Nuclear Force
The strong nuclear force has a short range, meaning it's effective only over very short distances. The presence of neutrons helps to extend the effective range of this force, leading to enhanced stability. This is particularly true for heavier atoms, where the electrostatic repulsion between many protons is significant.
Isotopes and Nuclear Stability
Isotopes are atoms of the same element with the same number of protons but a different number of neutrons. Different isotopes of the same element exhibit varying degrees of stability. Some isotopes are stable, while others are radioactive and undergo decay to achieve greater stability. This variation highlights the importance of the neutron-to-proton ratio in determining nuclear stability.
Conclusion: Stability Defined by Nucleon Numbers and the Strong Force
In conclusion, while many particles exist within and around the atom, only the number of protons and neutrons directly influence the stability of the atom. Electrons influence chemical reactivity but not nuclear stability. The strong nuclear force, influenced by the neutron-to-proton ratio, is the primary determinant of an atom's resistance to radioactive decay. Positrons, neutrinos, and other subatomic particles play a negligible role in the stability of typical atoms. Understanding the intricate balance of forces within the nucleus is crucial to comprehending the fascinating diversity of atomic stability across the periodic table. This fundamental understanding serves as a cornerstone for advancements in nuclear physics, chemistry, and numerous related fields.
Latest Posts
Latest Posts
-
The Letters F I F O Refer To
Mar 20, 2025
-
The Following Are Advantages To Group Decision Making Except Blank
Mar 20, 2025
-
A Factor That Causes Overhead Costs Is Called A
Mar 20, 2025
-
Management Acounting Focuses On Estimeated Future
Mar 20, 2025
-
Steven Roberts Oregon Mental Health Counselor
Mar 20, 2025
Related Post
Thank you for visiting our website which covers about Which Particles Do Not Affect The Stability Of The Atom . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
