Provide The Major Organic Product Of The Following Reaction
Holbox
Mar 24, 2025 · 6 min read
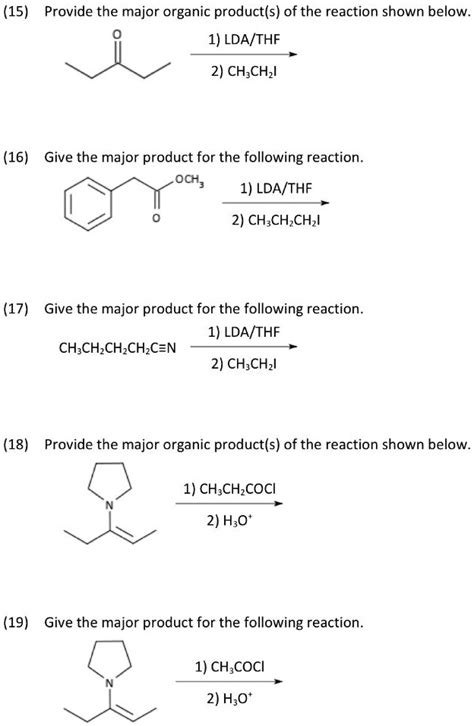
Table of Contents
- Provide The Major Organic Product Of The Following Reaction
- Table of Contents
- Predicting the Major Organic Product: A Deep Dive into Reaction Mechanisms
- Understanding Reaction Mechanisms: The Key to Prediction
- Predicting Products: Case Studies
- Factors Affecting Product Distribution
- Conclusion
- Latest Posts
- Latest Posts
- Related Post
Predicting the Major Organic Product: A Deep Dive into Reaction Mechanisms
Predicting the major organic product of a given reaction is a cornerstone of organic chemistry. This skill requires a thorough understanding of reaction mechanisms, functional group transformations, and the factors influencing reaction selectivity. This article will explore various reaction types, focusing on predicting the major product, encompassing stereochemistry and regiochemistry where applicable. We'll delve into several examples, highlighting the reasoning behind our predictions and the nuances that often determine the major product.
Understanding Reaction Mechanisms: The Key to Prediction
Before tackling specific reactions, it's crucial to grasp the concept of reaction mechanisms. A reaction mechanism describes the step-by-step process of bond breaking and bond formation during a chemical transformation. Understanding the mechanism allows us to predict the intermediate structures and, ultimately, the final product. Different mechanisms lead to different products, and even slight variations in conditions can alter the pathway. Key mechanistic concepts include:
- Nucleophilic Attack: A nucleophile, an electron-rich species, attacks an electrophile, an electron-deficient species. This is a fundamental step in many reactions.
- Electrophilic Attack: An electrophile attacks a nucleophile. This is common in reactions involving alkenes and aromatic compounds.
- Carbocation Rearrangements: Carbocations, positively charged carbon atoms, are highly reactive intermediates that can undergo rearrangements to form more stable carbocations. This rearrangement profoundly influences the final product.
- SN1 vs. SN2 Reactions: These nucleophilic substitution reactions differ in their mechanisms and stereochemistry, leading to different products. SN1 reactions proceed via a carbocation intermediate, often resulting in racemization, while SN2 reactions are concerted and lead to inversion of configuration.
- E1 vs. E2 Eliminations: These elimination reactions also differ in mechanism and stereochemistry. E1 reactions proceed via a carbocation intermediate, while E2 reactions are concerted and often require a specific anti-periplanar geometry.
Predicting Products: Case Studies
Let's examine several reaction types and predict their major organic products, emphasizing the underlying reasoning.
1. SN1 Reaction: Tertiary Alkyl Halide Hydrolysis
Reaction: Hydrolysis of tert-butyl bromide in aqueous ethanol.
Mechanism: The tert-butyl bromide undergoes an SN1 reaction. The C-Br bond breaks heterolytically, forming a tert-butyl carbocation. Water acts as a nucleophile, attacking the carbocation, followed by deprotonation to yield the alcohol.
Major Product: tert-butyl alcohol. The tertiary carbocation is relatively stable, favoring the SN1 pathway. The product is racemic due to the planar nature of the carbocation intermediate.
2. SN2 Reaction: Primary Alkyl Halide with Strong Nucleophile
Reaction: Reaction of methyl bromide with sodium cyanide (NaCN) in DMF.
Mechanism: This is a classic SN2 reaction. The cyanide ion acts as a strong nucleophile, attacking the methyl carbon from the backside, causing inversion of configuration.
Major Product: Acetonitrile (methyl cyanide). The strong nucleophile and primary alkyl halide favor the SN2 pathway, leading to a clean inversion of stereochemistry.
3. E1 Elimination: Tertiary Alkyl Halide Dehydration
Reaction: Dehydration of tert-butyl alcohol with concentrated sulfuric acid.
Mechanism: The alcohol is protonated by sulfuric acid, forming a good leaving group (water). Loss of water forms a tertiary carbocation, which then undergoes deprotonation to yield an alkene.
Major Product: Isobutylene (2-methylpropene). The more substituted alkene is the major product, following Zaitsev's rule, which states that the most substituted alkene is generally the most stable and therefore the major product.
4. E2 Elimination: Secondary Alkyl Halide with Strong Base
Reaction: Reaction of 2-bromobutane with potassium tert-butoxide (t-BuOK) in tert-butanol.
Mechanism: This is an E2 elimination reaction. The bulky base abstracts a proton from the β-carbon, while the bromide ion leaves simultaneously, forming a double bond. The stereochemistry must be anti-periplanar for the reaction to proceed efficiently.
Major Product: 2-butene (predominantly the trans isomer). Zaitsev's rule applies here, favoring the more substituted alkene. The stereochemistry of the starting material influences the stereochemistry of the product, with the anti-periplanar arrangement leading to the trans isomer as the major product.
5. Electrophilic Aromatic Substitution: Nitration of Benzene
Reaction: Nitration of benzene with nitric acid and sulfuric acid.
Mechanism: The nitronium ion (NO2+) acts as an electrophile, attacking the benzene ring. After a rearrangement, a proton is lost, regenerating the aromatic system.
Major Product: Nitrobenzene. Benzene is highly reactive towards electrophilic substitution, and the nitronium ion is a strong electrophile. Only one product is formed due to the symmetry of the benzene ring.
6. Addition Reactions: Bromination of Alkenes
Reaction: Addition of bromine (Br2) to cyclohexene.
Mechanism: Bromine adds across the double bond in an anti-addition manner. The alkene acts as a nucleophile, attacking one bromine atom, forming a cyclic bromonium ion intermediate. A bromide ion then attacks the bromonium ion from the opposite side, resulting in anti-addition.
Major Product: 1,2-dibromocyclohexane (trans isomer). The anti-addition mechanism dictates the stereochemistry of the product.
7. Grignard Reaction: Addition to a Ketone
Reaction: Reaction of phenylmagnesium bromide (Grignard reagent) with acetone.
Mechanism: The Grignard reagent acts as a nucleophile, attacking the carbonyl carbon of acetone. After protonation, a tertiary alcohol is formed.
Major Product: 2-phenyl-2-propanol. The Grignard reagent adds to the carbonyl carbon, resulting in the formation of a new carbon-carbon bond.
8. Aldol Condensation: Reaction of Two Aldehydes
Reaction: Aldol condensation of acetaldehyde.
Mechanism: One molecule of acetaldehyde acts as a nucleophile, attacking the carbonyl carbon of another molecule. After dehydration, an α,β-unsaturated aldehyde is formed.
Major Product: Crotonaldehyde (but-2-enal). The aldol condensation forms a carbon-carbon bond and creates a conjugated system.
Factors Affecting Product Distribution
Several factors can influence the product distribution in organic reactions:
- Steric Hindrance: Bulky groups can hinder the approach of reactants, affecting reaction rates and selectivity.
- Electronic Effects: Electron-donating or electron-withdrawing groups can influence the reactivity of functional groups.
- Temperature: Temperature affects the relative rates of competing reactions.
- Solvent: The solvent can influence the stability of intermediates and transition states.
- Catalyst: Catalysts can accelerate reactions and alter the reaction pathway.
Conclusion
Predicting the major organic product requires a solid understanding of reaction mechanisms, functional group transformations, and the various factors that influence reaction selectivity. By carefully analyzing the reactants, reaction conditions, and the underlying mechanism, one can reliably predict the major product. This article provides a foundation for mastering this essential skill in organic chemistry. Continued practice and exposure to diverse reactions will enhance one's predictive capabilities, ultimately leading to a deeper comprehension of organic chemistry principles. Remember that while these examples illustrate common outcomes, there can always be exceptions and minor side products formed, highlighting the complexity and beauty of organic chemistry. A thorough understanding of the principles outlined here will provide a strong basis for navigating these complexities.
Latest Posts
Latest Posts
-
Which Of The Following Inequalities Matches The Graph
Mar 26, 2025
-
Based On The Values In Cells A51 A55 What Formula
Mar 26, 2025
-
When Supplies Are Purchased On Credit It Means That
Mar 26, 2025
-
P Is The Insured On A Participating Life Policy
Mar 26, 2025
-
On July 1 A Company Receives An Invoice
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about Provide The Major Organic Product Of The Following Reaction . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
