Name The Following Organic Compounds Chegg
Holbox
Mar 16, 2025 · 6 min read
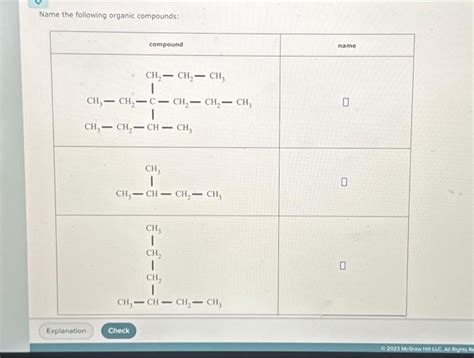
Table of Contents
Name the Following Organic Compounds: A Comprehensive Guide
Naming organic compounds might seem daunting at first, but with a systematic approach and understanding of fundamental principles, it becomes a manageable and even enjoyable task. This comprehensive guide will walk you through the process, covering various functional groups and complexities, providing you with the tools to confidently name a wide range of organic molecules. We will delve into the intricacies of IUPAC nomenclature, the internationally accepted standard for naming organic compounds.
Understanding the IUPAC System
The International Union of Pure and Applied Chemistry (IUPAC) established a standardized system for naming organic compounds to ensure clarity and consistency across the scientific community. This system is based on identifying the longest continuous carbon chain (parent chain), identifying functional groups, and numbering the carbon atoms in the chain. Understanding these key aspects is crucial for accurate naming.
Identifying the Parent Chain
The parent chain is the longest continuous chain of carbon atoms in the molecule. It forms the base name of the compound. For example, in a molecule containing a six-carbon chain, the base name would be "hexane." If there are multiple chains of equal length, the one with the most substituents is chosen as the parent chain.
Identifying Functional Groups
Functional groups are specific atoms or groups of atoms within a molecule that determine its chemical properties and reactivity. These groups are given priority in the naming process. Common functional groups include:
-
Alkanes: Single bonds between carbon atoms (e.g., methane, ethane, propane). These are the simplest hydrocarbons.
-
Alkenes: Contain at least one carbon-carbon double bond (e.g., ethene, propene). The suffix "-ene" indicates the presence of a double bond.
-
Alkynes: Contain at least one carbon-carbon triple bond (e.g., ethyne, propyne). The suffix "-yne" indicates the presence of a triple bond.
-
Alcohols: Contain a hydroxyl group (-OH) attached to a carbon atom (e.g., methanol, ethanol). The suffix "-ol" indicates the presence of an alcohol group. The position of the hydroxyl group is indicated by a number.
-
Aldehydes: Contain a carbonyl group (-CHO) at the end of a carbon chain (e.g., methanal, ethanal). The suffix "-al" indicates an aldehyde.
-
Ketones: Contain a carbonyl group (=O) within the carbon chain (e.g., propanone, butanone). The suffix "-one" indicates a ketone. The position of the carbonyl group is indicated by a number.
-
Carboxylic Acids: Contain a carboxyl group (-COOH) at the end of a carbon chain (e.g., methanoic acid, ethanoic acid). The suffix "-oic acid" indicates a carboxylic acid.
-
Amines: Contain an amino group (-NH2) (e.g., methylamine, ethylamine). The suffix "-amine" indicates an amine.
Numbering the Carbon Atoms
The carbon atoms in the parent chain are numbered to indicate the position of substituents and functional groups. Numbering starts from the end of the chain that gives the substituents the lowest possible numbers. If there are multiple substituents, the numbering is chosen to give the lowest possible combination of numbers.
Naming Substituents
Substituents are groups of atoms attached to the parent chain. They are named according to their structure and position. Common substituents include alkyl groups (e.g., methyl, ethyl, propyl) and halogen atoms (e.g., fluoro, chloro, bromo, iodo). The position of a substituent is indicated by a number.
Examples of Naming Organic Compounds
Let's work through some examples to illustrate the process:
Example 1: CH3CH2CH2CH3
This is a four-carbon alkane. The longest continuous chain is four carbons long, so the base name is butane. There are no substituents or functional groups.
Name: Butane
Example 2: CH3CH=CHCH3
This molecule contains a four-carbon chain with a double bond between carbons 2 and 3. The base name is butene. The position of the double bond is indicated by the number 2 (but-2-ene).
Name: But-2-ene
Example 3: CH3CH2CH2OH
This is a three-carbon chain with a hydroxyl group (-OH) on carbon 1. The base name is propane, and the presence of the hydroxyl group indicates it is an alcohol. The suffix "-ol" is added, and the position of the hydroxyl group is indicated (propan-1-ol).
Name: Propan-1-ol
Example 4: CH3COCH3
This molecule has a three-carbon chain with a carbonyl group (=O) on carbon 2. The base name is propane, and the carbonyl group within the chain indicates it is a ketone. The suffix "-one" is added, and the position of the carbonyl group is indicated (propan-2-one). This compound is also commonly known as acetone.
Name: Propan-2-one
Example 5: CH3CH2COOH
This molecule has a three-carbon chain with a carboxyl group (-COOH) at the end. The base name is propane, and the carboxyl group indicates a carboxylic acid. The suffix "-oic acid" is added.
Name: Propanoic acid
Example 6: More Complex Structures
Consider a molecule with multiple substituents: CH3CH(CH3)CH2CH(Cl)CH3.
-
Identify the parent chain: The longest continuous carbon chain is five carbons, making the base name pentane.
-
Number the carbons: Numbering from left to right gives the substituents the lowest numbers (2-methyl, 4-chloro).
-
Name the substituents: A methyl group (-CH3) is at carbon 2, and a chloro group (-Cl) is at carbon 4.
-
Combine the names: The complete name is 4-chloro-2-methylpentane.
Name: 4-chloro-2-methylpentane
Advanced Naming Conventions
The examples above represent simpler structures. More complex molecules require a deeper understanding of IUPAC nomenclature, including:
-
Cyclic Compounds: Compounds containing rings of carbon atoms require specific naming conventions, often involving prefixes like "cyclo" (e.g., cyclohexane).
-
Aromatic Compounds: Aromatic compounds, like benzene derivatives, have their own unique naming rules based on the benzene ring as the parent structure.
-
Stereoisomers: Molecules with the same molecular formula but different spatial arrangements (e.g., cis-trans isomers) require additional prefixes to specify their stereochemistry.
-
Polyfunctional Compounds: Molecules containing multiple functional groups require a prioritized order in naming, with some functional groups taking precedence over others.
Tips for Mastering Organic Compound Naming
-
Practice Regularly: The best way to master organic compound naming is through consistent practice. Work through numerous examples, starting with simple structures and gradually progressing to more complex ones.
-
Use Nomenclature Resources: Various online resources, textbooks, and educational websites offer extensive practice problems and tutorials on IUPAC nomenclature.
-
Break Down Complex Molecules: When faced with a complex molecule, break it down into its constituent parts (parent chain, substituents, functional groups) and apply the rules systematically.
-
Understand the Logic: Focus on understanding the underlying principles of IUPAC nomenclature rather than memorizing rules. The system is logical and follows consistent patterns.
-
Seek Clarification: Don't hesitate to seek help if you encounter difficulties. Consult textbooks, online resources, or ask for assistance from instructors or peers.
By diligently following these steps and dedicating time to practice, you can build a solid understanding of IUPAC nomenclature and confidently name a vast array of organic compounds. Remember, the key is to approach each molecule systematically, identifying the parent chain, functional groups, substituents, and numbering the carbons accordingly. With practice and patience, this skill will become second nature.
Latest Posts
Latest Posts
-
Strategic Implementation Is Thought To Be
Mar 17, 2025
-
Laker Company Reported The Following January
Mar 17, 2025
-
Quantitative Analysis Of Vinegar Via Titration
Mar 17, 2025
-
The Interest Rate A Company Pays On 1 Year 5 Year
Mar 17, 2025
-
The Shape Of An Atomic Orbital Is Associated With
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Name The Following Organic Compounds Chegg . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
