Quantitative Analysis Of Vinegar Via Titration
Holbox
Mar 17, 2025 · 7 min read
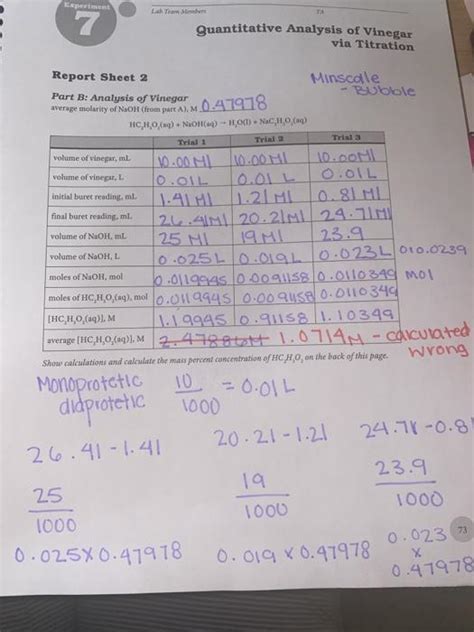
Table of Contents
Quantitative Analysis of Vinegar via Titration: A Comprehensive Guide
Vinegar, a staple in kitchens worldwide, is essentially a dilute solution of acetic acid (CH₃COOH) in water. Its characteristic sour taste and pungent aroma stem directly from this acetic acid content. Determining the precise concentration of acetic acid in vinegar is crucial for various applications, from ensuring product quality control in the manufacturing process to conducting scientific experiments requiring a known concentration. This is where quantitative analysis, specifically titration, comes into play. This comprehensive guide delves into the quantitative analysis of vinegar via titration, exploring the underlying principles, procedures, and associated calculations.
Understanding Titration: The Basics
Titration is a fundamental analytical technique used to determine the concentration of an unknown solution (analyte) by reacting it with a solution of known concentration (titrant). This reaction proceeds until the equivalence point is reached – the point where the moles of titrant added are stoichiometrically equivalent to the moles of analyte present. In the case of vinegar analysis, the analyte is the acetic acid in the vinegar, and the titrant is usually a standardized solution of a strong base, such as sodium hydroxide (NaOH).
The Chemistry Behind the Reaction
The titration of acetic acid with sodium hydroxide is an acid-base neutralization reaction:
CH₃COOH(aq) + NaOH(aq) → CH₃COONa(aq) + H₂O(l)
Acetic acid, a weak acid, reacts with sodium hydroxide, a strong base, to produce sodium acetate, a salt, and water. The reaction is essentially a proton transfer from the acetic acid molecule to the hydroxide ion.
Choosing the Right Indicator
To visually determine the equivalence point, an indicator is added to the analyte solution. Indicators are substances that change color depending on the pH of the solution. Phenolphthalein is a common choice for this titration because its color change occurs within the pH range of the equivalence point. Phenolphthalein is colorless in acidic solutions and pink in basic solutions. The equivalence point is reached when a single drop of NaOH causes a persistent pink color to appear in the solution.
The Titration Procedure: A Step-by-Step Guide
The accurate determination of acetic acid concentration requires meticulous attention to detail throughout the titration process. Here's a step-by-step guide:
1. Preparing the Materials and Solutions:
- Standardized NaOH solution: A solution of sodium hydroxide with accurately known concentration. This is typically prepared and standardized before the titration using a primary standard, such as potassium hydrogen phthalate (KHP).
- Vinegar sample: A precisely measured volume of the vinegar sample to be analyzed.
- Phenolphthalein indicator: A few drops of phenolphthalein are added to the vinegar sample.
- Burette: A calibrated glass tube used to dispense the NaOH solution.
- Erlenmeyer flask: A conical flask to hold the vinegar sample.
- Pipette: Used for accurate measurement of the vinegar sample.
- Wash bottle: Filled with distilled water for rinsing.
2. Setting up the Titration:
- Rinse the burette with the standardized NaOH solution to ensure no residual water affects the concentration. Fill the burette with the NaOH solution, ensuring that there are no air bubbles in the delivery tube.
- Record the initial burette reading accurately.
- Using a pipette, accurately measure a known volume of the vinegar sample (e.g., 10.00 mL) and transfer it to the Erlenmeyer flask.
- Add a few drops of phenolphthalein indicator to the vinegar sample.
3. Performing the Titration:
- Slowly add the NaOH solution from the burette to the vinegar sample while constantly swirling the flask. The solution will initially remain colorless.
- As the equivalence point approaches, the addition of NaOH should be slowed down significantly, adding the solution drop-wise.
- The endpoint is reached when a single drop of NaOH causes a persistent pale pink color to appear in the solution, indicating that the equivalence point has been reached.
4. Recording the Data:
- Record the final burette reading accurately.
- The volume of NaOH used is the difference between the initial and final burette readings.
Calculations and Data Analysis
Once the titration is complete, the concentration of acetic acid in the vinegar sample can be calculated using the following formula:
Molarity of Acetic Acid (Mₐ) = (Molarity of NaOH (Mₙ) × Volume of NaOH (Vₙ) ) / Volume of Vinegar (Vₐ)
Where:
- Mₐ is the molarity of acetic acid in the vinegar sample (mol/L).
- Mₙ is the molarity of the standardized NaOH solution (mol/L).
- Vₙ is the volume of NaOH solution used in the titration (L).
- Vₐ is the volume of the vinegar sample used in the titration (L).
The molar mass of acetic acid (CH₃COOH) is 60.05 g/mol. To calculate the percentage of acetic acid by weight in the vinegar, you can use the following formula:
% Acetic Acid by weight = (Mₐ × Molar mass of CH₃COOH × Vₐ) / Mass of Vinegar Sample × 100%
This calculation considers the molarity of acetic acid, its molar mass, the volume of the vinegar sample used, and the total mass of the vinegar sample. The result provides the percentage of acetic acid by weight present in the vinegar sample.
Sources of Error and Accuracy Improvement
Several factors can influence the accuracy of the titration results. Understanding and minimizing these sources of error is crucial for obtaining reliable data.
Potential Sources of Error:
- Impurities in the NaOH solution: The presence of impurities in the standardized NaOH solution can affect its actual concentration, leading to inaccurate results.
- Improper standardization of NaOH: An inaccurate standardization of the NaOH solution will propagate errors throughout the titration.
- Incorrect reading of the burette: Errors in reading the initial and final burette readings can significantly impact the calculated volume of NaOH used.
- Indicator error: The indicator itself may exhibit a slight delay in its color change, causing a slight deviation from the true equivalence point.
- Parallax error: Incorrect observation of the meniscus level in the burette can lead to parallax error.
Improving Accuracy and Precision:
- Multiple titrations: Performing multiple titrations and calculating the average value significantly improves the precision and reduces the effect of random errors.
- Proper cleaning and rinsing: Thoroughly cleaning and rinsing all glassware with distilled water prevents contamination and ensures accurate measurements.
- Careful observation of the endpoint: Precise observation of the endpoint minimizes indicator error.
- Slow addition of titrant near the endpoint: Gradual addition of the titrant near the endpoint allows for better control and prevents overshooting the equivalence point.
- Use of calibrated glassware: Using calibrated glassware ensures accurate measurement of volumes.
Advanced Techniques and Applications
While the standard titration method described above is effective, more sophisticated techniques can be used for enhanced accuracy and analysis of other components in vinegar.
Potentiometric Titration
Potentiometric titration uses a pH meter to monitor the pH changes during the titration. This method provides a more precise determination of the equivalence point, eliminating the subjective nature of visual indicators. The pH data can be plotted against the volume of titrant added, yielding a titration curve. The equivalence point is identified as the steepest point of the curve.
Determining Other Components in Vinegar
Vinegar is a complex mixture, and titration can be adapted to determine the concentration of other components. For example, the concentration of various mineral acids or other organic acids present in vinegar can be determined using appropriate titrants and indicators.
Applications in Quality Control
The quantitative analysis of vinegar via titration plays a vital role in quality control within the food industry. It ensures that the vinegar meets the required acetic acid concentration, maintaining consistency and meeting regulatory standards.
Conclusion
Quantitative analysis of vinegar via titration provides a straightforward yet powerful method for determining the acetic acid concentration. This technique is widely applicable in various settings, from educational laboratories to industrial quality control. By understanding the underlying principles, meticulously following the procedures, and carefully analyzing the data, one can accurately determine the acetic acid content of vinegar with high precision and reliability. Moreover, by understanding and mitigating potential sources of error, the reliability and accuracy of the obtained results are significantly enhanced. Through mastering this technique, researchers and professionals alike gain a crucial tool for evaluating the quality and properties of this ubiquitous household and industrial substance.
Latest Posts
Latest Posts
-
A Toy Rocket Is Launched Vertically From Ground Level
Mar 17, 2025
-
Experiment 34 An Equilibrium Constant Pre Lab Answers
Mar 17, 2025
-
The Preferred Way To Avoid An Ethnocentric Perspective Is To
Mar 17, 2025
-
Which Of The Following Best Describes Evolution
Mar 17, 2025
-
Noncellular Infectious Protein Particles Are Called
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Quantitative Analysis Of Vinegar Via Titration . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
