The Shape Of An Atomic Orbital Is Associated With
Holbox
Mar 17, 2025 · 6 min read
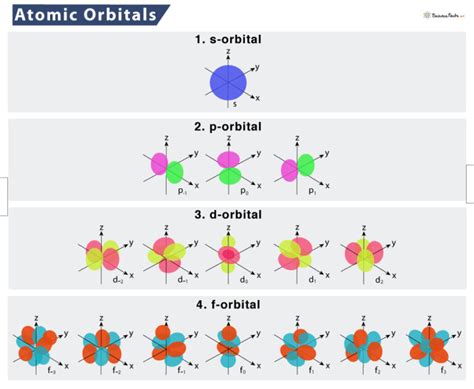
Table of Contents
The Shape of an Atomic Orbital is Associated With: A Deep Dive into Electron Probability Density
The shape of an atomic orbital is a crucial concept in chemistry, underpinning our understanding of chemical bonding, molecular geometry, and the properties of matter. It's not a simple matter of a defined boundary like a planet's orbit; instead, it's a representation of the probability of finding an electron within a specific region of space around an atom's nucleus. This probability is described by a mathematical function called a wave function, and its square gives us the electron density. Understanding this relationship is key to comprehending the intricacies of atomic structure.
The Quantum Mechanical Model and Atomic Orbitals
Unlike the simplistic Bohr model, the quantum mechanical model provides a more accurate and nuanced description of the atom. It depicts electrons not as orbiting particles in defined paths, but as existing in regions of space called atomic orbitals, characterized by their specific energy levels and shapes. These orbitals are defined by three quantum numbers:
-
Principal Quantum Number (n): This determines the energy level of the electron and the size of the orbital. Higher values of n indicate higher energy levels and larger orbitals. n can be any positive integer (1, 2, 3...).
-
Azimuthal Quantum Number (l): This determines the shape of the orbital and is related to the orbital angular momentum. l can have integer values from 0 to n - 1. Each value of l corresponds to a subshell:
- l = 0: s subshell (spherical shape)
- l = 1: p subshell (dumbbell shape)
- l = 2: d subshell (more complex shapes)
- l = 3: f subshell (even more complex shapes)
-
Magnetic Quantum Number (ml): This determines the spatial orientation of the orbital within a subshell. ml can have integer values from -l to +l, including 0. For example, a p subshell (l = 1) has three orbitals (ml = -1, 0, +1), oriented along the x, y, and z axes, respectively.
The Shapes of Atomic Orbitals: A Detailed Look
The shapes of atomic orbitals are crucial in determining how atoms interact and bond with each other. Let's explore the common shapes in more detail:
s Orbitals
s orbitals (l = 0) are spherically symmetrical around the nucleus. This means the probability of finding the electron is the same at any given distance from the nucleus in any direction. The size of the s orbital increases as the principal quantum number (n) increases. Thus, a 1s orbital is smaller than a 2s orbital, which is smaller than a 3s orbital, and so on. The higher energy levels have additional radial nodes (regions of zero electron density).
p Orbitals
p orbitals (l = 1) have a dumbbell shape with two lobes oriented along a specific axis (x, y, or z). There's a node at the nucleus, meaning the probability of finding the electron at the nucleus is zero. Each p subshell contains three p orbitals, designated as px, py, and pz, each oriented along a different Cartesian axis. The size of the p orbitals, like s orbitals, increases with increasing n.
d Orbitals
d orbitals (l = 2) have more complex shapes than s and p orbitals. A d subshell contains five d orbitals, with various shapes including cloverleaf and doughnut-like structures. These orbitals have multiple nodes, both radial and angular. Their orientations and shapes are important for understanding the formation of coordination complexes and other advanced chemical phenomena.
f Orbitals
f orbitals (l = 3) exhibit even more intricate shapes, with seven orbitals in a subshell. Their complex geometries are crucial for understanding the behavior of elements in the f-block (lanthanides and actinides). Due to their complexity, we often focus on the general overall shape rather than the specifics of individual f orbitals.
Factors Influencing Orbital Shape: Beyond the Quantum Numbers
While the quantum numbers primarily define orbital shape, other factors subtly influence the electron density distribution:
-
Electron-Electron Repulsion: The presence of multiple electrons in an atom causes them to repel each other, affecting the electron cloud distribution and slightly distorting the idealized shapes of the orbitals. This is particularly pronounced in atoms with many electrons.
-
Shielding Effect: Inner electrons shield outer electrons from the full positive charge of the nucleus. This shielding effect influences the effective nuclear charge experienced by the outer electrons, affecting the size and shape of the orbitals. Outer electrons are less strongly attracted to the nucleus and are more diffuse than they would be without shielding.
-
Penetration Effect: The penetration effect describes the ability of an electron in a particular orbital to approach the nucleus. Electrons in s orbitals have a higher penetration effect than those in p orbitals, which have a higher penetration effect than those in d orbitals, and so on. This means s electrons are more strongly attracted to the nucleus and experience a higher effective nuclear charge.
The Significance of Orbital Shape in Chemical Bonding
The shapes of atomic orbitals are fundamentally important for understanding how atoms form chemical bonds. The overlap of atomic orbitals between atoms leads to the formation of molecular orbitals, which describe the electron density in the molecule.
-
Sigma (σ) Bonds: These are formed by the head-on overlap of atomic orbitals, such as the overlap of two s orbitals or an s orbital and a p orbital. Sigma bonds are strong and are found in most molecules.
-
Pi (π) Bonds: These are formed by the side-on overlap of atomic orbitals, such as the overlap of two p orbitals. Pi bonds are weaker than sigma bonds and are found in molecules with double or triple bonds.
The specific shapes of the atomic orbitals dictate the orientation and strength of these bonds, influencing the molecular geometry and reactivity of the molecule. For example, the linear geometry of molecules like CO2 is a direct result of the sigma and pi bonding interactions involving the p orbitals of the carbon and oxygen atoms.
Beyond the Basics: Hybridization and Molecular Orbital Theory
To fully understand molecular structure and bonding, we need to delve into more advanced concepts:
-
Hybridization: This theory explains how atomic orbitals can mix to form hybrid orbitals with different shapes and energies, optimized for bonding. For example, sp3 hybridization in methane (CH4) creates four equivalent sp3 hybrid orbitals that point towards the corners of a tetrahedron, maximizing electron-electron repulsion and resulting in the tetrahedral geometry.
-
Molecular Orbital Theory: This theory goes beyond simple atomic orbital overlap and considers the combination of atomic orbitals to form molecular orbitals that encompass the entire molecule. This approach provides a more sophisticated and accurate description of bonding, particularly for molecules with delocalized electrons, like benzene.
Conclusion: The Ever-Evolving Understanding of Atomic Orbitals
The shape of an atomic orbital is inextricably linked to its electron probability density, and this relationship is governed by the quantum numbers and other subtle electronic interactions. These shapes are not merely abstract mathematical constructs but fundamental to understanding chemical bonding, molecular geometry, and the properties of matter. From simple s orbitals to the more complex d and f orbitals, the diversity of shapes highlights the rich complexity of atomic structure and the power of quantum mechanics in explaining the world around us. As our understanding of quantum mechanics deepens, our models of atomic orbitals will continue to evolve, offering a continually more refined picture of the atom's intricate and beautiful internal structure. This ongoing refinement will undoubtedly lead to new insights and advancements in chemistry and related fields. The study of atomic orbitals, therefore, is a dynamic and ever-evolving field with profound implications for our understanding of the universe.
Latest Posts
Latest Posts
-
Banks Typically Come Under Financial Stress Because Of
Mar 17, 2025
-
Choose All That Are Characteristics Of Neural Pools
Mar 17, 2025
-
The Two Topics Of Primary Concern In Macroeconomics Are
Mar 17, 2025
-
A Toy Rocket Is Launched Vertically From Ground Level
Mar 17, 2025
-
Experiment 34 An Equilibrium Constant Pre Lab Answers
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about The Shape Of An Atomic Orbital Is Associated With . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
