Choose The Best Lewis Structure For Icl5
Holbox
Mar 17, 2025 · 4 min read
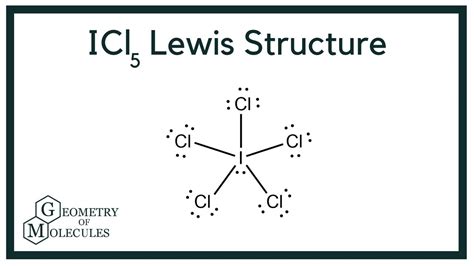
Table of Contents
Choosing the Best Lewis Structure for ICl₅: A Comprehensive Guide
The determination of the best Lewis structure for iodine pentachloride (ICl₅) involves understanding valence electrons, formal charges, and the principles of minimizing formal charge to achieve the most stable structure. While multiple Lewis structures might initially seem possible, only one truly represents the most stable and accurate depiction of ICl₅'s bonding. This article will guide you through the process, exploring different possibilities and ultimately arriving at the optimal Lewis structure.
Understanding Lewis Structures and VSEPR Theory
Before diving into ICl₅ specifically, let's review the fundamentals. A Lewis structure, also known as an electron dot structure, is a visual representation of the valence electrons of atoms within a molecule. These structures show how atoms are bonded together and illustrate the lone pairs of electrons present. Accurate Lewis structures are crucial for predicting molecular geometry and properties.
The Valence Shell Electron Pair Repulsion (VSEPR) theory complements Lewis structures. VSEPR theory predicts the three-dimensional arrangement of atoms in a molecule based on the repulsion between electron pairs (both bonding and non-bonding). This theory is essential for determining the molecular shape, bond angles, and polarity.
Determining Valence Electrons for ICl₅
To construct the Lewis structure, we first need to determine the total number of valence electrons involved.
- Iodine (I): Iodine is in Group 17 (or VIIA) of the periodic table, meaning it has 7 valence electrons.
- Chlorine (Cl): Chlorine, also in Group 17, has 7 valence electrons each. Since there are five chlorine atoms, this contributes 5 * 7 = 35 valence electrons.
Therefore, the total number of valence electrons in ICl₅ is 7 + 35 = 42.
Possible Lewis Structures and Formal Charge Calculations
While several arrangements might initially seem plausible, let's examine them systematically, calculating formal charges to assess their stability. The formal charge is the difference between the number of valence electrons in an isolated atom and the number of electrons assigned to that atom in a Lewis structure. A lower formal charge generally indicates a more stable structure.
Structure 1: Iodine as the Central Atom with Five Single Bonds and One Lone Pair
This structure attempts to distribute the 42 valence electrons using five single bonds between iodine and the five chlorine atoms, and one lone pair on the iodine atom.
Cl
|
Cl-I-Cl
|
Cl
|
Cl
- Formal Charge Calculation:
- Iodine: 7 (valence electrons) - (6 bonding electrons + 2 non-bonding electrons) = -1
- Each Chlorine: 7 - (2 bonding electrons + 6 non-bonding electrons) = 0
This structure has a -1 formal charge on the iodine atom. While not inherently impossible, it's not ideal because it places a negative charge on the less electronegative atom.
Structure 2: Iodine as the Central Atom with Five Single Bonds and Zero Lone Pairs
This structure assigns all 42 valence electrons to five single bonds between iodine and five chlorine atoms, leaving no lone pairs on iodine. This structure is not viable because it violates the octet rule for iodine, exceeding the number of electrons it can accommodate in its valence shell. Iodine, being a period 5 element, can exhibit expanded octets, but this doesn't mean it should be arbitrarily overfilled.
Structure 3: Explores structures with double or triple bonds
Creating double or triple bonds to attempt to satisfy the octet rule for all atoms leads to excessively high formal charges, making these structures highly improbable. These structures are unfavorable and should be discarded based on the principle of minimizing formal charge.
The Best Lewis Structure for ICl₅
Based on the analysis of the possible structures and the formal charge calculations, the most stable and accurate Lewis structure for ICl₅ is Structure 1: Iodine as the central atom with five single bonds to five chlorine atoms and one lone pair on iodine.
Although it features a -1 formal charge on the iodine atom, this is the least unfavorable arrangement. The alternative structures either violate the octet rule (though iodine can exhibit hypervalency) or result in significantly higher formal charges.
Therefore, the optimal Lewis Structure is:
Cl
|
Cl-I-Cl
|
Cl
|
Cl
Molecular Geometry and Polarity of ICl₅
Using VSEPR theory, we can determine the molecular geometry of ICl₅. The central iodine atom is surrounded by five bonding pairs and one lone pair, resulting in an octahedral electron geometry. However, the lone pair distorts the geometry, leading to a square pyramidal molecular geometry. This shape is slightly distorted due to the lone pair occupying more space.
Furthermore, ICl₅ is a polar molecule. Although the individual I-Cl bonds might be considered somewhat polar, the overall molecular geometry leads to an uneven distribution of electron density, resulting in a net dipole moment.
Conclusion: The Importance of Formal Charge Minimization and VSEPR Theory
Choosing the best Lewis structure for ICl₅ exemplifies the importance of considering both valence electrons and formal charges. While iodine can have more than eight electrons in its valence shell, minimizing formal charges is crucial for predicting molecular geometry and properties. The use of VSEPR theory helps in establishing the three-dimensional structure and its influence on polarity. The best Lewis structure, as outlined, is the one that best balances these factors. Understanding these concepts is essential for accurately representing molecular structures and predicting their properties.
Latest Posts
Latest Posts
-
Strategic Implementation Is Thought To Be
Mar 17, 2025
-
Laker Company Reported The Following January
Mar 17, 2025
-
Quantitative Analysis Of Vinegar Via Titration
Mar 17, 2025
-
The Interest Rate A Company Pays On 1 Year 5 Year
Mar 17, 2025
-
The Shape Of An Atomic Orbital Is Associated With
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Choose The Best Lewis Structure For Icl5 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
