At What Temperature Does Solid Turn To Liquid
Holbox
Mar 14, 2025 · 5 min read
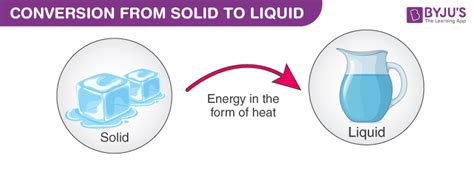
Table of Contents
At What Temperature Does Solid Turn to Liquid? Understanding Melting Points and Phase Transitions
The seemingly simple question, "At what temperature does a solid turn to liquid?" belies a complex interplay of molecular forces, energy transfer, and material properties. While we often think of melting as a straightforward process, understanding the nuances requires delving into the fascinating world of phase transitions and thermodynamics. This article will explore the concept of melting points, the factors influencing them, and the various ways in which solids transition to liquids.
Understanding the Melting Point
The melting point (or liquefaction point) is the temperature at which a solid substance transitions into a liquid state. This transition is characterized by the absorption of energy, breaking the strong intermolecular forces holding the solid's rigid structure together. Crucially, this occurs at a specific temperature for a pure substance under standard pressure conditions. This constancy makes the melting point a vital physical property used for material identification and purity analysis.
The Role of Intermolecular Forces
The strength of the intermolecular forces within a solid directly impacts its melting point. Stronger intermolecular forces require more energy to overcome, resulting in a higher melting point. Consider the following examples:
- Ionic compounds: These compounds, like sodium chloride (NaCl), have strong electrostatic attractions between oppositely charged ions. This leads to very high melting points.
- Covalent network solids: Substances like diamond and quartz have strong covalent bonds forming a continuous network throughout the solid. These materials exhibit exceptionally high melting points.
- Metallic solids: Metallic bonds, a result of delocalized electrons, contribute to a wide range of melting points depending on the specific metal and the number of delocalized electrons.
- Molecular solids: These solids, formed by weaker intermolecular forces like van der Waals forces or hydrogen bonds, generally have much lower melting points. The weaker the intermolecular force, the lower the melting point.
Pressure's Influence on Melting Point
While temperature plays the dominant role in melting, pressure also exerts an influence, although often a less significant one. The effect of pressure depends on the substance's density change upon melting.
- Most substances: For most substances, the liquid phase is less dense than the solid phase. Increasing pressure therefore favors the denser solid phase, resulting in a higher melting point.
- Water: An Exception: Water is a notable exception. Ice is less dense than liquid water (hence ice floats). Therefore, increasing pressure on ice lowers its melting point. This unusual behavior has significant implications for glaciology and other fields.
Factors Affecting Melting Point
Beyond the intrinsic properties of the substance itself, several external factors can influence its melting point:
-
Purity: Impurities in a substance disrupt the regular crystal lattice structure, weakening the intermolecular forces and thus lowering the melting point. This principle is applied in techniques like cryoscopy, used to determine the purity of substances. The greater the impurity concentration, the greater the melting point depression.
-
Allotropes: Some elements exist in multiple forms, known as allotropes, with different crystal structures. These allotropes often exhibit different melting points. For instance, carbon exists as diamond and graphite, each having vastly different melting points due to their distinct structural arrangements.
-
Crystal Structure: The arrangement of atoms or molecules within the solid's crystal lattice significantly affects the melting point. A more ordered, tightly packed structure typically results in a higher melting point.
Types of Melting
Not all solids melt in the same way. Different types of melting behaviors exist, depending on the material's characteristics:
-
Congruent Melting: This is the most common type, where the solid melts directly into its liquid phase without any decomposition or phase changes. The composition of the solid remains the same throughout the melting process.
-
Incongruent Melting: This type involves decomposition of the solid into different phases before melting occurs. For example, some hydrated salts decompose to release water before melting completely. The composition of the resulting liquid is different from the original solid.
Beyond the Simple Melting Point: Advanced Concepts
The simple notion of a single melting point applies mainly to pure substances. Mixtures and complex materials exhibit more complicated melting behavior.
-
Melting Range: Impure substances typically melt over a range of temperatures, rather than at a single point. This is because the presence of impurities disrupts the crystal lattice, causing different regions of the solid to melt at slightly different temperatures.
-
Eutectic Point: In mixtures of two or more components, a eutectic point exists, representing the lowest melting temperature achievable for that specific mixture composition. At the eutectic point, the mixture melts completely at a single temperature.
-
Phase Diagrams: Phase diagrams graphically represent the relationships between temperature, pressure, and the phases of a substance. They are crucial for understanding melting behavior under various conditions, including the effects of pressure and composition.
Applications of Melting Point Knowledge
The melting point is a fundamental property with widespread applications across various fields:
-
Material Science: Understanding melting points is vital for material processing, alloy development, and the design of materials with specific properties.
-
Chemistry: Melting points are used for substance identification, purity analysis, and reaction monitoring.
-
Geology: Melting points of minerals are crucial in understanding geological processes like magma formation and volcanic eruptions.
-
Pharmaceutical Industry: Melting point determination is a critical quality control test in pharmaceutical manufacturing, ensuring drug purity and consistency.
Conclusion
The temperature at which a solid transforms into a liquid, its melting point, is not a simple matter of a single number. It's a rich area of study encompassing intermolecular forces, energy transfer, and the complex interplay of various external factors. Understanding the subtleties of melting points is essential across numerous scientific and industrial disciplines. By appreciating the influence of purity, pressure, crystal structure, and other factors, we gain a more profound understanding of the physical world and the properties of the materials that shape it. From the simple act of melting an ice cube to the complex processes involved in manufacturing advanced materials, the concept of the melting point remains a cornerstone of scientific inquiry and technological advancement.
Latest Posts
Latest Posts
-
A Circuit Is Constructed With 5 Capacitors And A Battery
Mar 14, 2025
-
Identify The Stages Of Meiosis On The Diagram
Mar 14, 2025
-
The Objective Of Standard Cost Variance Analysis Is
Mar 14, 2025
-
Net Marketplaes Amy Either Support Contractual
Mar 14, 2025
-
You Have Two Capacitors One With Capacitance
Mar 14, 2025
Related Post
Thank you for visiting our website which covers about At What Temperature Does Solid Turn To Liquid . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
