A Buffer Is A Substance That
Holbox
Mar 16, 2025 · 6 min read
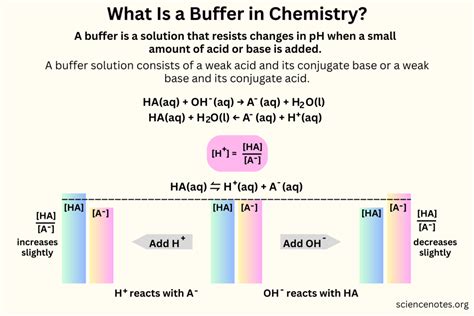
Table of Contents
A Buffer is a Substance That… Resists Change! Understanding Buffer Solutions in Chemistry
A buffer is a substance that resists changes in pH upon the addition of small amounts of acid or base. This crucial property makes buffers indispensable in numerous chemical and biological systems, ensuring stability in environments sensitive to pH fluctuations. Understanding how buffers work, their applications, and the factors influencing their effectiveness is critical for anyone studying chemistry, biology, or related fields. This comprehensive guide delves into the intricacies of buffer solutions, providing a thorough understanding of their nature and function.
What is a Buffer Solution?
A buffer solution, or simply a buffer, is an aqueous solution consisting of a mixture of a weak acid and its conjugate base, or a weak base and its conjugate acid. This unique composition allows the buffer to neutralize small additions of either strong acid or strong base, thereby minimizing changes in pH. The key to understanding buffer function lies in the equilibrium between the weak acid/base and its conjugate.
The Chemistry Behind Buffer Action
The effectiveness of a buffer stems from Le Chatelier's principle. This principle states that if a change of condition is applied to a system in equilibrium, the system will shift in a direction that relieves the stress. When a strong acid is added to a buffer solution containing a weak acid and its conjugate base, the added H⁺ ions react with the conjugate base, forming more of the weak acid. This reaction consumes the added H⁺ ions, preventing a significant decrease in pH. Similarly, when a strong base is added, the OH⁻ ions react with the weak acid, forming more of the conjugate base, thus minimizing the pH increase.
Types of Buffer Solutions
Several types of buffer solutions exist, each tailored for specific applications based on their pH range and buffering capacity. The most common types include:
1. Acidic Buffers
Acidic buffers are prepared using a weak acid and its conjugate base salt. Examples include:
- Acetic acid/sodium acetate buffer: This is a commonly used buffer with a pH range around 4.76.
- Citric acid/sodium citrate buffer: This buffer is effective in a slightly lower pH range.
- Phosphoric acid/phosphate buffer: This offers a wider pH range due to phosphoric acid's multiple dissociation constants.
2. Basic Buffers
Basic buffers are prepared using a weak base and its conjugate acid salt. Examples include:
- Ammonia/ammonium chloride buffer: This buffer is effective in a pH range around 9.25.
- Tris-HCl buffer (Tris(hydroxymethyl)aminomethane hydrochloride): Frequently used in biological applications, maintaining a relatively stable pH in the slightly alkaline range.
3. Biological Buffers
Many biological systems rely on naturally occurring buffers to maintain a stable internal environment. Important examples include:
- Phosphate buffer system: Crucial in maintaining the pH of blood and intracellular fluids.
- Bicarbonate buffer system: Plays a significant role in regulating blood pH.
- Protein buffers: Proteins, with their various ionizable groups, contribute to buffering capacity within cells.
Factors Affecting Buffer Capacity
The effectiveness of a buffer isn't unlimited. Several factors influence its capacity to resist pH changes:
1. Concentration of the Buffer Components
A higher concentration of the weak acid and its conjugate base results in a greater buffering capacity. The buffer can absorb more added acid or base before a significant pH shift occurs. This is because a higher concentration provides more reactive species to neutralize the added strong acid or base.
2. Ratio of Acid to Conjugate Base
The buffer's pH is determined by the ratio of the weak acid to its conjugate base, as described by the Henderson-Hasselbalch equation:
pH = pKa + log([A⁻]/[HA])
where:
- pH is the solution's pH
- pKa is the negative logarithm of the acid dissociation constant (Ka) of the weak acid
- [A⁻] is the concentration of the conjugate base
- [HA] is the concentration of the weak acid
The most effective buffering occurs when the ratio of [A⁻]/[HA] is close to 1, meaning the concentrations of the acid and conjugate base are approximately equal. This corresponds to the pH being equal to the pKa of the weak acid. Deviation from this ideal ratio reduces the buffer's effectiveness.
3. The Strength of the Acid or Base
The strength of the weak acid or base significantly impacts the buffer's capacity. A weaker acid or base will provide less effective buffering compared to a stronger one, meaning a larger pH change will occur with the addition of a strong acid or base. This is because weaker acids or bases dissociate to a lesser extent, resulting in fewer species available to neutralize the added strong acid or base.
4. Temperature
Temperature affects the dissociation constant (Ka) of the weak acid, consequently influencing the buffer's pH and effectiveness. Changes in temperature can shift the equilibrium, leading to altered buffering capacity.
Applications of Buffer Solutions
The ability of buffers to maintain a stable pH has led to their widespread application across various fields:
1. Biological Systems
Buffers are essential for maintaining the pH of biological systems. Without them, even small changes in acidity or alkalinity could disrupt crucial biological processes. Examples include:
- Maintaining blood pH: The bicarbonate buffer system is critical for keeping blood pH within a narrow range (7.35-7.45). Deviations from this range can be life-threatening.
- Enzyme activity: Many enzymes function optimally within a specific pH range. Buffers help maintain this optimal pH, ensuring proper enzyme activity.
- Cell culture: Buffers are used in cell culture media to maintain the pH required for cell growth and survival.
2. Chemical Analysis
Buffers are frequently used in chemical analysis to control the pH of solutions during titrations, precipitation reactions, and other analytical procedures. Controlling the pH is crucial for ensuring accurate and reliable results. For example, the pH must be controlled during many spectrophotometric analyses to ensure that the measured absorbance is directly proportional to the concentration of the analyte.
3. Industrial Processes
Buffers are employed in various industrial processes where pH control is vital. Examples include:
- Pharmaceutical industry: Buffers are used in the formulation of drugs and medications to maintain stability and prevent degradation.
- Food industry: Buffers are used to control the pH of food products, improving their quality, shelf life, and taste.
- Textile industry: Buffers are used to control the pH in dyeing and finishing processes.
4. Environmental Monitoring
Buffers are used in environmental monitoring to maintain the pH of samples collected for analysis, preventing changes in pH that could affect the accuracy of measurements. This is particularly important when dealing with sensitive environmental indicators.
Calculating Buffer pH and Capacity
The Henderson-Hasselbalch equation is crucial for calculating the pH of a buffer solution. Furthermore, the buffer capacity, which represents the amount of acid or base a buffer can neutralize before a significant pH change occurs, can be estimated. While precise calculations require more complex methods, a general understanding of the principles allows for practical application. This is particularly important in designing buffer solutions for specific applications.
Conclusion
Buffers are essential substances that maintain a relatively constant pH even with the addition of small amounts of acid or base. This remarkable ability is rooted in the equilibrium between a weak acid and its conjugate base (or a weak base and its conjugate acid), leveraging Le Chatelier's principle to resist pH changes. Their importance spans various fields, including biology, chemistry, industry, and environmental science, highlighting the critical role they play in maintaining stability and controlling chemical reactions across diverse applications. A deep understanding of buffer solutions, their properties, and limitations is fundamental to many scientific and technological endeavors.
Latest Posts
Latest Posts
-
Quantitative Analysis Of Vinegar Via Titration
Mar 17, 2025
-
The Interest Rate A Company Pays On 1 Year 5 Year
Mar 17, 2025
-
The Shape Of An Atomic Orbital Is Associated With
Mar 17, 2025
-
Parallelism In Writing Can Reflect Which Of The Following
Mar 17, 2025
-
Which Of The Following Is A Ball And Socket Joint
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about A Buffer Is A Substance That . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
