Write The Balanced Chemical Equation For The Reaction Shown
Holbox
Mar 26, 2025 · 6 min read
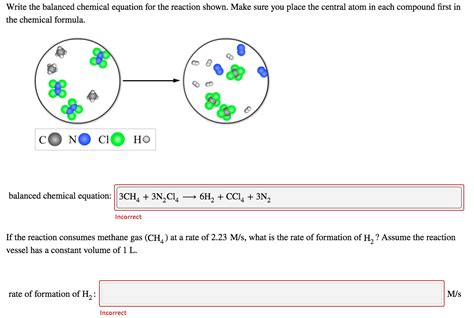
Table of Contents
- Write The Balanced Chemical Equation For The Reaction Shown
- Table of Contents
- Writing Balanced Chemical Equations: A Comprehensive Guide
- Understanding Chemical Equations
- Balancing Chemical Equations: A Step-by-Step Approach
- 1. Identify the Reactants and Products
- 2. Write the Unbalanced Equation
- 3. Count the Atoms
- 4. Balance the Equation
- 5. Ensure Coefficients are in the Lowest Whole Number Ratio
- Examples of Balancing Chemical Equations
- Advanced Techniques and Considerations
- Conclusion
- Latest Posts
- Latest Posts
- Related Post
Writing Balanced Chemical Equations: A Comprehensive Guide
Writing a balanced chemical equation is a fundamental skill in chemistry. It's more than just writing down the reactants and products; it's about representing the quantitative relationships between them, ensuring that the law of conservation of mass is upheld. This means the number of atoms of each element must be the same on both sides of the equation – the reactants and the products. This article will guide you through the process, from identifying reactants and products to mastering techniques for balancing complex equations.
Understanding Chemical Equations
A chemical equation is a symbolic representation of a chemical reaction. It uses chemical formulas to depict the reactants (starting materials) and products (resulting substances). The reactants are written on the left side of an arrow, while the products are written on the right. The arrow indicates the direction of the reaction.
For example, the combustion of methane (CH₄) in oxygen (O₂) to produce carbon dioxide (CO₂) and water (H₂O) is represented as:
CH₄ + O₂ → CO₂ + H₂O
This equation, however, is unbalanced. Notice that the number of oxygen atoms isn't the same on both sides. Balancing this equation is crucial for understanding the stoichiometry (quantitative relationships) of the reaction.
Balancing Chemical Equations: A Step-by-Step Approach
Balancing a chemical equation is a trial-and-error process, but with systematic approaches, you can master it. Here’s a step-by-step guide:
1. Identify the Reactants and Products
Clearly identify all reactants and products involved in the chemical reaction. This usually involves understanding the chemical reaction's description.
2. Write the Unbalanced Equation
Write the unbalanced chemical equation using the correct chemical formulas for each reactant and product. Remember to use subscripts correctly to indicate the number of atoms of each element in a molecule.
3. Count the Atoms
Carefully count the number of atoms of each element on both sides of the equation (reactants and products). Use a table to organize this information – this makes the balancing process much easier to manage, especially with more complex equations.
4. Balance the Equation
This is the core of the process. You need to adjust the coefficients (numbers placed in front of the chemical formulas) to equalize the number of atoms of each element on both sides of the equation. Never change the subscripts within the chemical formulas – doing so changes the chemical identity of the substance.
- Start with the most complex molecule: Begin by balancing the element that appears in the most complex molecule (the molecule with the most atoms).
- Balance one element at a time: Focus on balancing one element at a time, and avoid juggling too many coefficients simultaneously.
- Balance polyatomic ions as units: If polyatomic ions (like sulfate, SO₄²⁻, or nitrate, NO₃⁻) remain unchanged throughout the reaction, treat them as single units. This simplifies the balancing process.
- Check your work: After adjusting each coefficient, recount the atoms on both sides to ensure they are balanced.
5. Ensure Coefficients are in the Lowest Whole Number Ratio
Once you've balanced the equation, ensure that the coefficients are in the lowest whole number ratio. If all coefficients can be divided by a common factor, divide them to obtain the simplest ratio.
Examples of Balancing Chemical Equations
Let's work through some examples to solidify your understanding:
Example 1: Combustion of Methane
The unbalanced equation:
CH₄ + O₂ → CO₂ + H₂O
-
Count the atoms:
- Reactants: 1 C, 4 H, 2 O
- Products: 1 C, 2 H, 3 O
-
Balance the equation:
- Start with hydrogen: We have 4 H on the left and 2 H on the right. Add a coefficient of 2 in front of H₂O:
CH₄ + O₂ → CO₂ + 2H₂O
- Now let's balance the oxygen: we have 2 O on the left and 4 O on the right (2 from CO₂ and 2 from 2H₂O). We need to add a coefficient of 2 in front of O₂:
CH₄ + 2O₂ → CO₂ + 2H₂O
- Now recount the atoms: 1 C, 4 H, 4 O on both sides. The equation is balanced.
Example 2: Reaction of Iron with Oxygen
The unbalanced equation:
Fe + O₂ → Fe₂O₃
-
Count the atoms:
- Reactants: 1 Fe, 2 O
- Products: 2 Fe, 3 O
-
Balance the equation:
- Start with iron: Add a coefficient of 2 in front of Fe:
2Fe + O₂ → Fe₂O₃
- Now balance the oxygen: We have 2 O on the left and 3 O on the right. To balance the oxygen atoms, we need to use fractional coefficients, which is acceptable at this intermediate step. Let's add a coefficient of 3/2 in front of O₂:
2Fe + (3/2)O₂ → Fe₂O₃
- To get rid of the fraction, multiply all coefficients by 2:
4Fe + 3O₂ → 2Fe₂O₃
- Recounting the atoms confirms a balanced equation.
Example 3: A More Complex Reaction
Let's consider the reaction between propane (C₃H₈) and oxygen:
C₃H₈ + O₂ → CO₂ + H₂O
-
Count the atoms:
- Reactants: 3 C, 8 H, 2 O
- Products: 1 C, 2 H, 3 O
-
Balance the equation:
- Balance Carbon: Add a coefficient of 3 in front of CO₂:
C₃H₈ + O₂ → 3CO₂ + H₂O
- Balance Hydrogen: Add a coefficient of 4 in front of H₂O:
C₃H₈ + O₂ → 3CO₂ + 4H₂O
- Balance Oxygen: We have 2 O on the left and 10 O on the right (6 from 3CO₂ and 4 from 4H₂O). Add a coefficient of 5 in front of O₂:
C₃H₈ + 5O₂ → 3CO₂ + 4H₂O
- The equation is now balanced.
Advanced Techniques and Considerations
-
Redox Reactions: Balancing redox reactions (reactions involving electron transfer) often requires a different approach. Methods like the half-reaction method are commonly used to balance these more complex equations.
-
Ionic Equations: For reactions occurring in aqueous solutions, ionic equations show the reaction in terms of ions. Balancing ionic equations requires balancing both the charge and the mass.
-
Combustion Analysis: The process of balancing combustion reactions, where a substance reacts with oxygen, often involves a trial-and-error approach focusing on balancing carbon and hydrogen first, followed by oxygen.
-
Practice Makes Perfect: Mastering the art of balancing chemical equations takes practice. Work through numerous examples, varying in complexity, to improve your skill and speed. The more you practice, the more efficient and confident you'll become.
Conclusion
Balancing chemical equations is a critical skill for anyone studying or working with chemistry. It's not just about manipulating numbers; it's about understanding the fundamental principle of the conservation of mass. By following the step-by-step approach outlined here and practicing regularly, you can confidently balance even complex chemical equations. Remember to always double-check your work and ensure the coefficients represent the lowest whole number ratio. With consistent practice and attention to detail, this essential skill will become second nature.
Latest Posts
Latest Posts
-
1 3 Butadiene Undergoes An Electrophilic Addition With Hbr
Mar 30, 2025
-
When We Say That Momentum Is Conserved We Mean
Mar 30, 2025
-
You Will Be Given To Complete An Aba Assignment
Mar 30, 2025
-
Peptidoglycan Is A Unique Macromolecule Found In Bacterial
Mar 30, 2025
-
Select The Five Major Mechanisms Of Antimicrobial Resistance
Mar 30, 2025
Related Post
Thank you for visiting our website which covers about Write The Balanced Chemical Equation For The Reaction Shown . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
