Which Polymers Are Composed Of Amino Acids
Holbox
Mar 25, 2025 · 5 min read
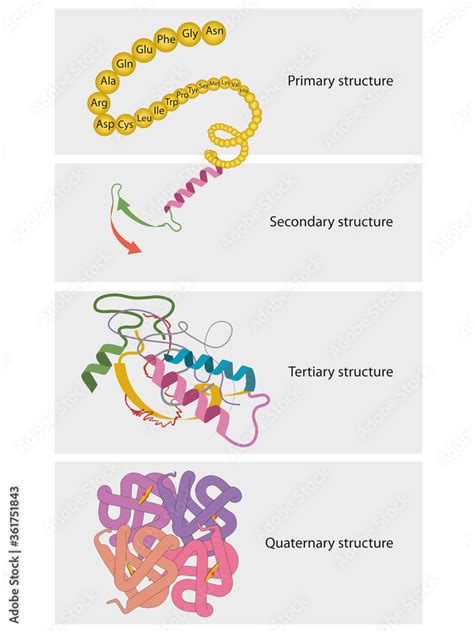
Table of Contents
- Which Polymers Are Composed Of Amino Acids
- Table of Contents
- Which Polymers are Composed of Amino Acids?
- The Building Blocks: Amino Acids
- Essential vs. Non-Essential Amino Acids
- Peptide Bonds: Linking Amino Acids
- Polypeptide Chain Structure
- Protein Structure: From Primary to Quaternary
- 1. Primary Structure
- 2. Secondary Structure
- 3. Tertiary Structure
- 4. Quaternary Structure
- Protein Function: A Diverse Landscape
- Post-Translational Modifications
- Studying Polypeptides and Proteins: Techniques and Approaches
- Conclusion: The Significance of Amino Acid Polymers
- Latest Posts
- Latest Posts
- Related Post
Which Polymers are Composed of Amino Acids?
Polymers composed of amino acids are known as polypeptides or proteins. While the terms are often used interchangeably, there's a subtle distinction: polypeptides refer to the linear chains of amino acids, while proteins describe the functional three-dimensional structures formed by one or more polypeptide chains. This article will delve into the fascinating world of these biopolymers, exploring their composition, structure, function, and the diverse roles they play in living organisms.
The Building Blocks: Amino Acids
The fundamental units of polypeptides and proteins are amino acids. These are organic molecules characterized by a central carbon atom (the α-carbon) bonded to four groups:
- An amino group (-NH₂): This group is basic and carries a positive charge at physiological pH.
- A carboxyl group (-COOH): This group is acidic and carries a negative charge at physiological pH.
- A hydrogen atom (-H): This is a simple, non-reactive group.
- A side chain (R-group): This is the variable group, and its structure defines the specific properties of each amino acid.
There are twenty standard amino acids commonly found in proteins, each with a unique R-group that contributes to the overall protein structure and function. These R-groups can be nonpolar (hydrophobic), polar (hydrophilic), positively charged (basic), or negatively charged (acidic). This diversity is crucial for the vast array of functions proteins perform.
Essential vs. Non-Essential Amino Acids
Amino acids are classified as either essential or non-essential:
- Essential amino acids: These cannot be synthesized by the human body and must be obtained from the diet. Examples include lysine, leucine, isoleucine, valine, methionine, phenylalanine, threonine, tryptophan, and histidine.
- Non-essential amino acids: These can be synthesized by the body from other metabolites. Examples include alanine, asparagine, aspartic acid, cysteine, glutamic acid, glutamine, glycine, proline, serine, and tyrosine.
The distinction between essential and non-essential is important for nutrition and understanding dietary requirements.
Peptide Bonds: Linking Amino Acids
Amino acids are linked together by peptide bonds, which are amide bonds formed between the carboxyl group of one amino acid and the amino group of another. This reaction involves the removal of a water molecule (dehydration synthesis). The resulting chain of amino acids is called a polypeptide. The sequence of amino acids in a polypeptide is its primary structure, and this sequence dictates the higher-order structures.
Polypeptide Chain Structure
A polypeptide chain has a specific directionality: one end has a free amino group (the N-terminus) and the other end has a free carboxyl group (the C-terminus). The sequence of amino acids is read from the N-terminus to the C-terminus.
Protein Structure: From Primary to Quaternary
The three-dimensional structure of a protein is crucial for its function. Proteins are organized into four levels of structure:
1. Primary Structure
This refers to the linear sequence of amino acids in a polypeptide chain. This sequence is determined by the genetic code and is crucial for determining the higher-order structures. Even a single amino acid substitution can significantly alter the protein's function, as seen in sickle cell anemia, where a single amino acid change in hemoglobin causes the protein to misfold.
2. Secondary Structure
This refers to local folding patterns within the polypeptide chain, stabilized by hydrogen bonds between the backbone atoms (the amino and carboxyl groups). Common secondary structures include:
- α-helices: A coiled structure resembling a spring.
- β-sheets: Extended structures formed by hydrogen bonds between adjacent polypeptide segments.
- Loops and turns: Irregular regions connecting α-helices and β-sheets.
The specific arrangement of secondary structures contributes to the overall three-dimensional shape of the protein.
3. Tertiary Structure
This refers to the overall three-dimensional arrangement of a single polypeptide chain, including the spatial relationships between the secondary structure elements. Tertiary structure is stabilized by various interactions, including:
- Hydrophobic interactions: Nonpolar side chains cluster together in the protein's interior, away from the surrounding water.
- Hydrogen bonds: Form between polar side chains.
- Ionic bonds (salt bridges): Form between oppositely charged side chains.
- Disulfide bonds: Covalent bonds between cysteine residues.
The tertiary structure determines the protein's function.
4. Quaternary Structure
This refers to the arrangement of multiple polypeptide chains (subunits) to form a functional protein. Not all proteins have a quaternary structure; some proteins function as single polypeptide chains. Quaternary structure is stabilized by the same interactions that stabilize tertiary structure. Examples of proteins with quaternary structure include hemoglobin and many enzymes.
Protein Function: A Diverse Landscape
Proteins are incredibly versatile and perform a vast array of functions in living organisms, including:
- Enzymes: Catalyze biochemical reactions.
- Structural proteins: Provide support and shape to cells and tissues (e.g., collagen, keratin).
- Transport proteins: Carry molecules across cell membranes (e.g., hemoglobin, membrane transporters).
- Motor proteins: Generate movement (e.g., myosin, kinesin).
- Hormones: Act as chemical messengers (e.g., insulin, glucagon).
- Antibodies: Part of the immune system, defending against pathogens.
- Receptors: Receive and transmit signals from the environment.
Post-Translational Modifications
After synthesis, proteins can undergo various modifications that affect their structure and function. These post-translational modifications include:
- Glycosylation: Addition of carbohydrate groups.
- Phosphorylation: Addition of phosphate groups.
- Ubiquitination: Addition of ubiquitin, often targeting proteins for degradation.
- Proteolytic cleavage: Removal of portions of the polypeptide chain.
These modifications can regulate protein activity, localization, and stability.
Studying Polypeptides and Proteins: Techniques and Approaches
Researchers use a variety of techniques to study polypeptides and proteins, including:
- Chromatography: Separates proteins based on their size, charge, or hydrophobicity.
- Electrophoresis: Separates proteins based on their size and charge.
- Mass spectrometry: Determines the mass and sequence of proteins.
- X-ray crystallography: Determines the three-dimensional structure of proteins.
- Nuclear magnetic resonance (NMR) spectroscopy: Determines the three-dimensional structure of proteins in solution.
Conclusion: The Significance of Amino Acid Polymers
Polypeptides and proteins, the polymers composed of amino acids, are fundamental to life. Their diverse structures and functions are essential for all biological processes. Understanding their composition, structure, and function is critical for advancing our knowledge in various fields, including medicine, biotechnology, and materials science. Further research continues to unveil the complexity and remarkable capabilities of these essential biomolecules. Ongoing studies explore protein folding, protein-protein interactions, and the development of novel therapeutic strategies targeting proteins implicated in disease. The field of proteomics, focused on the large-scale study of proteins, promises to further illuminate the intricate roles these molecules play in health and disease. The continuing exploration of the vast world of amino acid polymers promises exciting discoveries in the years to come.
Latest Posts
Latest Posts
-
P Is The Insured On A Participating Life Policy
Mar 26, 2025
-
On July 1 A Company Receives An Invoice
Mar 26, 2025
-
The Prokaryotic Cells That Built Stromatolites Are Classified As
Mar 26, 2025
-
A Nation Can Achieve Higher Economic Growth If
Mar 26, 2025
-
Experiment 3 Osmosis Direction And Concentration Gradients
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about Which Polymers Are Composed Of Amino Acids . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
