Experiment 3 Osmosis Direction And Concentration Gradients
Holbox
Mar 26, 2025 · 6 min read
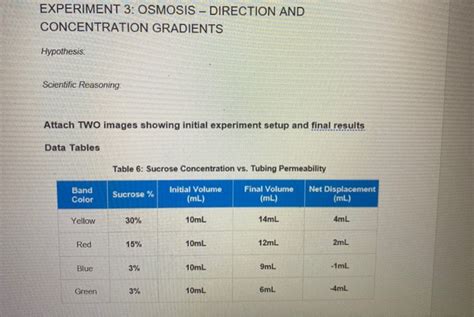
Table of Contents
- Experiment 3 Osmosis Direction And Concentration Gradients
- Table of Contents
- Experiment 3: Osmosis, Direction, and Concentration Gradients: A Deep Dive
- Understanding Osmosis: A Recap
- Key Terms to Remember:
- Experimental Setup: Investigating Osmosis
- Materials:
- Procedure:
- Results and Analysis: Interpreting the Data
- Expected Results:
- Data Analysis:
- Factors Affecting Osmosis: Beyond Concentration Gradients
- Temperature:
- Membrane Permeability:
- Surface Area:
- Pressure:
- Biological Significance of Osmosis: Real-World Applications
- Plant Cells:
- Animal Cells:
- Medical Applications:
- Experiment Variations and Extensions
- Conclusion: A Deeper Understanding of Osmosis
- Latest Posts
- Latest Posts
- Related Post
Experiment 3: Osmosis, Direction, and Concentration Gradients: A Deep Dive
Understanding osmosis is fundamental to comprehending biological processes at a cellular level. This experiment delves into the principles governing osmosis, specifically focusing on the direction of water movement and the influence of concentration gradients. We'll examine the process in detail, exploring experimental setups, results interpretation, and the broader implications of osmosis in various biological contexts.
Understanding Osmosis: A Recap
Osmosis is the passive movement of water molecules across a selectively permeable membrane from a region of higher water concentration to a region of lower water concentration. This movement continues until equilibrium is reached, meaning the water concentration is equal on both sides of the membrane. The selectively permeable membrane allows water molecules to pass through but restricts the movement of larger solute molecules. This selective permeability is crucial for maintaining cellular homeostasis.
Key Terms to Remember:
- Selectively permeable membrane: A membrane that allows certain molecules to pass through while restricting others. Cell membranes are prime examples.
- Water potential: The tendency of water to move from one area to another. It's influenced by solute concentration and pressure. High water potential means water tends to move out of that area; low water potential means water tends to move into that area.
- Solute potential: The component of water potential that is due to the presence of solutes. The more solute present, the lower the solute potential (and thus, the lower the overall water potential).
- Pressure potential: The component of water potential that is due to physical pressure. Turgor pressure in plant cells is an example of positive pressure potential.
- Isotonic solution: A solution with the same solute concentration as the cell's cytoplasm. There is no net movement of water.
- Hypotonic solution: A solution with a lower solute concentration than the cell's cytoplasm. Water moves into the cell.
- Hypertonic solution: A solution with a higher solute concentration than the cell's cytoplasm. Water moves out of the cell.
Experimental Setup: Investigating Osmosis
Several experimental setups can effectively demonstrate the principles of osmosis. A common method involves using dialysis tubing (a selectively permeable membrane) filled with a solution of varying concentrations and submerging it in a beaker containing a different solution.
Materials:
- Dialysis tubing
- Various sucrose solutions (e.g., 0%, 5%, 10%, 15%, 20%)
- Distilled water
- Beakers
- Graduated cylinders
- Balance
- Markers
Procedure:
- Prepare the dialysis tubing: Cut several pieces of dialysis tubing to equal lengths. Soak them in water to soften them and make them more permeable.
- Fill the tubing: Fill each piece of tubing with a different sucrose solution, ensuring that no air bubbles are trapped inside. Tie off one end securely with a knot.
- Weigh the bags: Carefully weigh each filled dialysis tubing bag using a balance. Record the initial weight.
- Submerge the bags: Submerge each bag in a separate beaker containing a known volume of distilled water. Ensure that the entire bag is submerged.
- Observe and measure: Observe the bags at regular intervals (e.g., every 30 minutes for several hours) and record any changes in appearance. At the end of the experiment, carefully remove the bags, gently blot them dry, and weigh them again. Record the final weight. Also measure the volume of water in each beaker.
Results and Analysis: Interpreting the Data
The results will clearly demonstrate the direction of water movement based on the concentration gradient.
Expected Results:
- Bags in hypotonic solutions: Bags filled with solutions of lower sucrose concentration (e.g., 0% or 5%) placed in distilled water will gain weight as water moves into them. They will become turgid (firm). The water level in the surrounding beaker will decrease.
- Bags in hypertonic solutions: Bags filled with solutions of higher sucrose concentration (e.g., 15% or 20%) placed in distilled water will lose weight as water moves out of them. They will become flaccid (limp). The water level in the surrounding beaker will increase.
- Bags in isotonic solutions: Bags filled with a solution of the same sucrose concentration as the surrounding water will show minimal change in weight.
Data Analysis:
Calculate the percentage change in mass for each dialysis tubing bag using the following formula:
Percentage change in mass = [(Final mass - Initial mass) / Initial mass] x 100
Plot the percentage change in mass against the initial sucrose concentration. This will visually represent the relationship between concentration gradient and water movement. Analyze the volume change in the surrounding beakers to confirm water movement.
Factors Affecting Osmosis: Beyond Concentration Gradients
While the concentration gradient is the primary driving force behind osmosis, other factors can influence the rate and direction of water movement.
Temperature:
Higher temperatures generally increase the rate of osmosis because water molecules possess more kinetic energy, leading to faster diffusion across the membrane.
Membrane Permeability:
The properties of the selectively permeable membrane itself play a crucial role. A more permeable membrane will allow for faster osmosis. Different membranes have varying degrees of permeability to water.
Surface Area:
A larger surface area of the membrane available for water movement will result in a faster rate of osmosis.
Pressure:
Applying pressure to one side of the membrane can influence the direction and rate of water movement. This is relevant in scenarios like reverse osmosis, where pressure is used to overcome the osmotic pressure and force water movement against the concentration gradient.
Biological Significance of Osmosis: Real-World Applications
Osmosis is a critical process for numerous biological functions:
Plant Cells:
Water uptake by plant roots relies heavily on osmosis. The concentration gradient between the soil water and the root cells drives water movement into the plant, leading to turgor pressure, which provides structural support. Wilting occurs when water loss through transpiration exceeds water uptake due to a hypertonic environment.
Animal Cells:
Maintaining proper hydration and electrolyte balance in animal cells is crucial for survival. Osmosis plays a significant role in regulating fluid balance in the body. For example, the kidneys regulate water reabsorption through osmosis, preventing excessive water loss or retention.
Medical Applications:
Understanding osmosis is essential in various medical applications. Intravenous solutions must be isotonic to prevent cell damage due to water influx or efflux. Dialysis treatments for kidney failure rely on principles of osmosis and diffusion to remove waste products from the blood.
Experiment Variations and Extensions
The basic osmosis experiment can be expanded upon to explore other aspects of the process. Here are a few examples:
- Using different solutes: Compare the effects of using different solutes (e.g., glucose, salts) of the same molarity. This can highlight the impact of solute properties on water potential.
- Investigating semipermeable membranes with different pore sizes: Explore how the pore size of the membrane affects the rate of osmosis.
- Quantifying osmotic pressure: Design an experiment to measure the osmotic pressure of different solutions using specialized equipment.
Conclusion: A Deeper Understanding of Osmosis
This experiment provides a practical understanding of osmosis, its driving force (the concentration gradient), and its significance in biological systems. By observing the movement of water across a selectively permeable membrane under controlled conditions, we can gain valuable insights into this fundamental biological process. This understanding is essential for grasping cellular physiology, plant biology, and numerous medical applications. Furthermore, exploring variations of this experiment allows for a more comprehensive understanding of the complex interplay of factors influencing osmosis. The knowledge gained through this detailed investigation can be applied in various biological contexts and has implications for many fields of scientific endeavor.
Latest Posts
Latest Posts
-
What Lists Can You Not Import From Excel Into Quickbooks
Mar 30, 2025
-
Triangle Congruence Theorems Common Core Geometry Homework Answer Key
Mar 30, 2025
-
Choose The Best Lewis Structure For So42
Mar 30, 2025
-
Forms Of Trust Include All Of The Following Except
Mar 30, 2025
-
Psychoanalytic Techniques Are Designed Primarily To Help Patients
Mar 30, 2025
Related Post
Thank you for visiting our website which covers about Experiment 3 Osmosis Direction And Concentration Gradients . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
