Choose The Best Lewis Structure For So42-
Holbox
Mar 30, 2025 · 5 min read
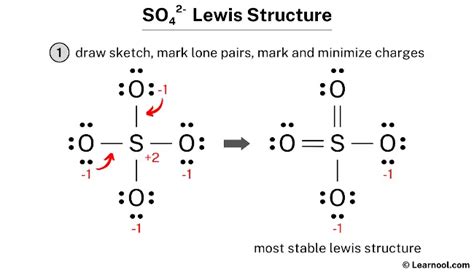
Table of Contents
- Choose The Best Lewis Structure For So42-
- Table of Contents
- Choosing the Best Lewis Structure for SO₄²⁻: A Deep Dive into Resonance and Formal Charge
- Understanding Lewis Structures and Their Limitations
- Key Concepts for Evaluating Lewis Structures
- Drawing Possible Lewis Structures for SO₄²⁻
- Evaluating the Structures Based on Formal Charge
- Resonance and the Best Lewis Structure for SO₄²⁻
- Conclusion: The Importance of Resonance and Formal Charge Minimization
- Latest Posts
- Latest Posts
- Related Post
Choosing the Best Lewis Structure for SO₄²⁻: A Deep Dive into Resonance and Formal Charge
The sulfate ion, SO₄²⁻, presents a classic example in chemistry for illustrating the concept of resonance structures and the importance of formal charge in selecting the best representation of a molecule's bonding. While several Lewis structures can be drawn for SO₄²⁻, not all are equally valid. This article will delve into the process of constructing and evaluating various Lewis structures for SO₄²⁻, ultimately determining the most accurate representation.
Understanding Lewis Structures and Their Limitations
A Lewis structure, also known as a Lewis dot diagram, is a simplified representation of a molecule's valence electrons and bonding. It shows how atoms are connected through single, double, or triple bonds, and indicates the presence of lone pairs of electrons. While extremely helpful in visualizing bonding, Lewis structures have limitations: they don't always accurately represent the true distribution of electrons in a molecule, particularly in cases involving resonance.
Key Concepts for Evaluating Lewis Structures
Before we start drawing structures for SO₄²⁻, let's review crucial concepts that guide our selection:
- Octet Rule: Most atoms strive to achieve a stable electron configuration with eight valence electrons (exceptions exist, like hydrogen and elements in the third row or beyond).
- Formal Charge: The formal charge of an atom in a Lewis structure is calculated as: Formal Charge = (Valence Electrons) - (Non-bonding Electrons) - ½(Bonding Electrons). A lower formal charge on each atom generally indicates a more stable structure.
- Resonance: When more than one valid Lewis structure can be drawn for a molecule, these structures are called resonance structures. The actual molecule is a hybrid of these resonance structures, meaning the electron distribution is a weighted average of all possible contributing structures.
Drawing Possible Lewis Structures for SO₄²⁻
Let's start by considering the valence electrons in SO₄²⁻:
- Sulfur (S) has 6 valence electrons.
- Oxygen (O) has 6 valence electrons each (x4 oxygen atoms = 24 valence electrons).
- The 2- charge adds 2 more electrons.
Total valence electrons = 6 + 24 + 2 = 32 electrons.
Now, let's explore some possible Lewis structures:
Structure 1: All Single Bonds
O
|
O-S-O
|
O
This structure places all four oxygen atoms singly bonded to the sulfur atom. Each oxygen atom has three lone pairs, and the sulfur atom has two lone pairs. All atoms satisfy the octet rule, except sulfur which will have 12 valence electrons. However, Sulfur is in the 3rd row and can expand it's octet.
Structure 2: One Double Bond
O
||
O-S-O
|
O
Here, one oxygen atom forms a double bond with sulfur. This structure still satisfies the octet rule for all atoms but introduces formal charges.
Structure 3: Two Double Bonds
O
||
O-S=O
||
O
This structure utilizes two double bonds, with formal charges being distributed.
Structure 4: Three Double Bonds (unlikely and highly unfavorable)
It is highly improbable to have three double bonds due to the excessive formal charges on this structure.
Evaluating the Structures Based on Formal Charge
Let's calculate the formal charges for each structure:
Structure 1 (All Single Bonds):
- Sulfur: 6 - 2 - ½(8) = 0
- Oxygen (single bonded): 6 - 6 - ½(2) = -1 (x3)
- Total charge: 0 + (-1) x 3 = -3. This is different from the -2 charge overall. In this case we must add an additional lone pair on Sulfur to ensure the overall charge matches the -2.
Structure 2 (One Double Bond):
- Sulfur: 6 - 2 - ½(10) = +1
- Oxygen (double bonded): 6 - 4 - ½(4) = 0
- Oxygen (single bonded): 6 - 6 - ½(2) = -1 (x2)
- Total charge: +1 + 0 + (-1) x 2 = -1. This is also incorrect.
Structure 3 (Two Double Bonds):
- Sulfur: 6 - 0 - ½(12) = 0
- Oxygen (double bonded): 6 - 4 - ½(4) = 0 (x2)
- Oxygen (single bonded): 6 - 6 - ½(2) = -1 (x2)
- Total charge: 0 + 0 x 2 + (-1) x 2 = -2. This structure is overall correct.
Structure 4 (Three Double Bonds): This structure would lead to significant formal charges on the sulfur and oxygen atoms, leading to its instability.
Resonance and the Best Lewis Structure for SO₄²⁻
While Structure 3 might seem like the best option, it's crucial to consider resonance. In reality, the SO₄²⁻ ion doesn't have two double bonds and two single bonds fixed in place. Instead, the electrons in the double bonds are delocalized across all four oxygen atoms. This means the actual structure is a hybrid of multiple resonance structures (including structures with only single bonds, one double bond etc.) . We can represent this delocalization using resonance structures and a resonance hybrid. The negative charges are effectively distributed equally among the four oxygen atoms.
All resonance structures contribute to the actual structure of the sulfate ion. However, those structures with the lowest formal charges are the most important contributors. In this case, the structure that minimizes formal charges is one with two double and two single bonds, although all formal charges should be accounted for and properly balanced to match the overall charge of -2.
Conclusion: The Importance of Resonance and Formal Charge Minimization
Choosing the "best" Lewis structure for SO₄²⁻ involves careful consideration of the octet rule, formal charges, and, most importantly, resonance. While no single Lewis structure perfectly represents the actual electron distribution, structures minimizing formal charges provide the most accurate picture. Understanding resonance is crucial for representing molecules with delocalized electrons, providing a far more realistic picture of molecular structure and properties. The SO₄²⁻ ion illustrates the power and limitations of the Lewis structure approach and underscores the need to consider resonance when dealing with complex molecules. The concept of resonance hybrids is necessary to represent the truer reality of electron distribution in the SO42- molecule. The formal charges of each resonance structure contribute to the understanding of stability and reactivity of the molecule.
Latest Posts
Latest Posts
-
The Following Picture Would Be Best Described As
Apr 01, 2025
-
Match Each Term With The Correct Definition
Apr 01, 2025
-
Match These Prefixes Suffixes And Roots To Their Meanings
Apr 01, 2025
-
Match The Bone With The Region It Comes From
Apr 01, 2025
-
The Anticodon Of A Particular Trna Molecule Is
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Choose The Best Lewis Structure For So42- . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
