What Is The Molar Mass Of Nh4 3po4
Holbox
Mar 16, 2025 · 5 min read
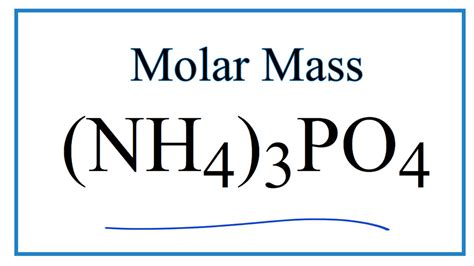
Table of Contents
What is the Molar Mass of (NH₄)₃PO₄? A Deep Dive into Chemical Calculations
Determining the molar mass of a compound is a fundamental concept in chemistry, crucial for various calculations and analyses. This article provides a comprehensive explanation of how to calculate the molar mass of ammonium phosphate, (NH₄)₃PO₄, also known as triammonium phosphate, and explores its significance in different chemical contexts. We'll delve into the step-by-step process, address common pitfalls, and highlight practical applications.
Understanding Molar Mass
Molar mass, expressed in grams per mole (g/mol), represents the mass of one mole of a substance. A mole is a unit representing Avogadro's number (approximately 6.022 x 10²³) of elementary entities, be it atoms, molecules, ions, or formula units. Essentially, the molar mass tells us the mass of 6.022 x 10²³ particles of a given substance.
Calculating molar mass involves summing the atomic masses of all the atoms present in a chemical formula. Atomic masses are typically obtained from the periodic table of elements. It's important to note that these atomic masses are weighted averages reflecting the isotopic composition of the element in nature.
Calculating the Molar Mass of (NH₄)₃PO₄
Ammonium phosphate, (NH₄)₃PO₄, is an ionic compound composed of three ammonium ions (NH₄⁺) and one phosphate ion (PO₄³⁻). To calculate its molar mass, we need to consider the atomic masses of the constituent elements: nitrogen (N), hydrogen (H), phosphorus (P), and oxygen (O).
Let's break down the calculation:
1. Identify the elements and their respective atomic masses:
- Nitrogen (N): Approximately 14.01 g/mol
- Hydrogen (H): Approximately 1.01 g/mol
- Phosphorus (P): Approximately 30.97 g/mol
- Oxygen (O): Approximately 16.00 g/mol
2. Determine the number of atoms of each element in the formula (NH₄)₃PO₄:
- Nitrogen (N): 3 ammonium ions x 1 nitrogen atom/ion = 3 nitrogen atoms
- Hydrogen (H): 3 ammonium ions x 4 hydrogen atoms/ion = 12 hydrogen atoms
- Phosphorus (P): 1 phosphorus atom
- Oxygen (O): 4 oxygen atoms
3. Calculate the molar mass contribution of each element:
- Nitrogen: 3 atoms x 14.01 g/mol/atom = 42.03 g/mol
- Hydrogen: 12 atoms x 1.01 g/mol/atom = 12.12 g/mol
- Phosphorus: 1 atom x 30.97 g/mol/atom = 30.97 g/mol
- Oxygen: 4 atoms x 16.00 g/mol/atom = 64.00 g/mol
4. Sum up the molar mass contributions of all elements:
Total molar mass of (NH₄)₃PO₄ = 42.03 g/mol + 12.12 g/mol + 30.97 g/mol + 64.00 g/mol = 149.12 g/mol
Therefore, the molar mass of ammonium phosphate, (NH₄)₃PO₄, is approximately 149.12 g/mol.
Significance and Applications of Molar Mass Calculations
The ability to accurately determine the molar mass of a compound has broad implications across numerous chemical disciplines. Some key applications include:
1. Stoichiometric Calculations:
Molar mass is fundamental to stoichiometry, the branch of chemistry dealing with quantitative relationships between reactants and products in chemical reactions. It allows us to convert between mass and moles, enabling precise predictions of reactant amounts needed or product yields expected in a reaction.
For example, if we know the mass of (NH₄)₃PO₄ used in a reaction, we can calculate the number of moles using the molar mass. This, in turn, allows us to determine the moles and ultimately the mass of other reactants or products involved.
2. Solution Preparation:
Molarity, a common unit of concentration, is defined as moles of solute per liter of solution. To prepare a solution of a specific molarity, we must first calculate the required mass of solute using its molar mass. This ensures the accurate preparation of solutions crucial for various experiments and analyses.
3. Determining Empirical and Molecular Formulas:
Molar mass plays a critical role in determining the empirical and molecular formulas of compounds. The empirical formula represents the simplest whole-number ratio of atoms in a compound, while the molecular formula represents the actual number of atoms of each element.
Knowing the molar mass allows us to determine the molecular formula from the empirical formula, particularly when experimental data provides the molar mass.
4. Gas Law Calculations:
The ideal gas law (PV = nRT) relates pressure (P), volume (V), number of moles (n), temperature (T), and the ideal gas constant (R). Calculating the number of moles (n) requires knowledge of the molar mass, allowing us to connect macroscopic properties of gases (P, V, T) to the microscopic quantities of molecules.
5. Analytical Chemistry:
Molar mass is crucial in many analytical techniques, including titration, gravimetric analysis, and spectrophotometry. Accurate molar mass determinations are essential for obtaining reliable results in these methods, which are used to determine the concentration or quantity of substances in samples.
6. Understanding Chemical Properties:
The molar mass, although not directly influencing chemical reactivity, helps contextualize observations. For instance, knowing the molar mass allows us to compare the relative masses of different reactants or products, providing insights into reaction stoichiometry and potentially explaining observed reaction rates or equilibrium positions.
Common Pitfalls in Molar Mass Calculations
While the concept is straightforward, several errors can occur during molar mass calculations:
-
Incorrect Formula: Using an incorrect chemical formula is the most common mistake. Double-check the formula to ensure its accuracy before starting calculations.
-
Incorrect Atomic Masses: Using outdated or inaccurate atomic masses from the periodic table can lead to significant errors. Refer to a reliable, up-to-date periodic table.
-
Mathematical Errors: Simple mathematical errors during the summation process can also affect the final result. Carefully review your calculations to avoid such errors.
-
Ignoring Subscripts and Coefficients: Pay close attention to subscripts (indicating the number of atoms of each element in a molecule) and coefficients (indicating the number of molecules in a reaction) to avoid miscounting atoms.
-
Unit Inconsistencies: Ensure consistent units throughout the calculation. For example, if you use atomic masses in grams per mole, the final result should also be in grams per mole.
Conclusion
Calculating the molar mass of a compound, such as (NH₄)₃PO₄, is a fundamental skill for any chemist or student of chemistry. This procedure, while seemingly simple, is critical for a wide range of applications across various chemical disciplines. By understanding the underlying principles and carefully avoiding potential pitfalls, one can accurately calculate molar masses and utilize them effectively in numerous chemical calculations and analyses. Accuracy is paramount, as errors in molar mass calculations can cascade into significant inaccuracies in subsequent analyses and experimental outcomes. Always double-check your work and ensure you are using the most up-to-date and reliable sources for atomic masses.
Latest Posts
Latest Posts
-
The Accounts In The Ledger Of Monroe Entertainment Co
Mar 17, 2025
-
A Process Cost Accounting System Is Most Appropriate When
Mar 17, 2025
-
An Example Of A Breach Of Ephi Is
Mar 17, 2025
-
What Is Involved In Safety Monitoring
Mar 17, 2025
-
Which Of The Following Best Describes The Term Cleavage
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about What Is The Molar Mass Of Nh4 3po4 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
