Put These Steps In The Mechanism Of Chymotrypsin Catalysis
Holbox
Mar 19, 2025 · 6 min read
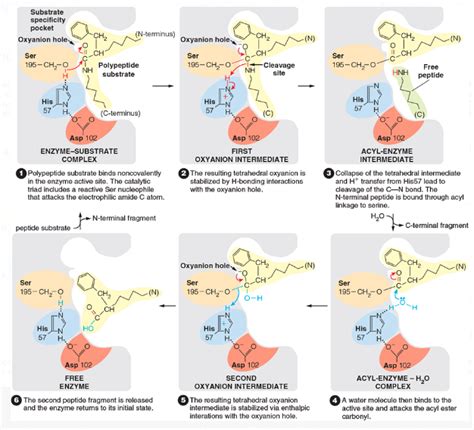
Table of Contents
The Mechanism of Chymotrypsin Catalysis: A Deep Dive
Chymotrypsin, a serine protease, is a fascinating enzyme renowned for its efficiency in catalyzing the hydrolysis of peptide bonds. Understanding its mechanism provides a compelling example of how enzymes achieve remarkable rate enhancements. This detailed exploration will dissect the catalytic mechanism of chymotrypsin, step-by-step, highlighting the key roles of its active site residues and the intricacies of its catalytic strategy.
Step 1: Substrate Binding and Specificity
The journey begins with the substrate binding to the enzyme's active site. Chymotrypsin exhibits a remarkable degree of specificity, primarily cleaving peptide bonds adjacent to large, hydrophobic amino acid residues such as phenylalanine, tyrosine, and tryptophan. This specificity is dictated by the enzyme's active site architecture.
The Active Site: A Pocket of Precision
The active site of chymotrypsin is a deep cleft, housing a crucial triad of amino acid residues: Serine 195, Histidine 57, and Aspartate 102. These three residues are strategically positioned to orchestrate the catalytic process. The hydrophobic pocket adjacent to Serine 195 is responsible for accommodating the large, nonpolar side chains of the target amino acids, ensuring the precise binding of the substrate. This pocket's shape and hydrophobicity are key determinants of the enzyme's substrate specificity.
Specificity Pocket: A Molecular Lock and Key
The specificity pocket is not merely a passive binding site; it actively participates in substrate recognition. Its shape and chemical properties dictate which amino acid side chains can fit and bind effectively. This "lock and key" interaction ensures that chymotrypsin preferentially interacts with and cleaves peptide bonds near bulky, hydrophobic amino acids, thereby maximizing catalytic efficiency and minimizing unwanted side reactions.
Step 2: Formation of the Tetrahedral Intermediate
Once the substrate is correctly positioned in the active site, the catalytic process begins. The crucial first step involves the nucleophilic attack by the hydroxyl group of Serine 195 on the carbonyl carbon of the peptide bond.
The Catalytic Triad in Action: Histidine's Pivotal Role
This nucleophilic attack is not spontaneous; it's facilitated by the catalytic triad. Histidine 57, acting as a general base, abstracts a proton from Serine 195's hydroxyl group, making it a much stronger nucleophile. This activated serine hydroxyl then attacks the carbonyl carbon of the peptide bond, forming a tetrahedral intermediate.
Aspartate 102: Stabilizing the Charge
Aspartate 102 plays a crucial role in stabilizing the positive charge that develops on the histidine residue during this proton transfer. This charge stabilization enhances the effectiveness of histidine's base catalysis, ensuring a smooth and efficient progression of the reaction. The positioning of the aspartate within the triad optimizes the interactions and facilitates the catalytic process.
Step 3: Collapse of the Tetrahedral Intermediate and Acyl-Enzyme Formation
The tetrahedral intermediate is inherently unstable. Its collapse leads to the formation of an acyl-enzyme intermediate.
Proton Transfer and Peptide Bond Cleavage
During the collapse, the bond between the carbonyl carbon and the nitrogen of the peptide bond is broken. His 57 now acts as a general acid, donating a proton to the nitrogen, which is then released as the amine portion of the cleaved peptide. The carboxyl group of the cleaved peptide remains temporarily attached to the serine residue through an ester linkage, forming the acyl-enzyme intermediate. This intermediate represents a crucial checkpoint in the reaction mechanism.
Step 4: Deacylation – Releasing the Second Product
The acyl-enzyme intermediate is not the final product. A second step, deacylation, is necessary to release the remaining portion of the original peptide.
Water as a Nucleophile
This step involves the entry of a water molecule into the active site. The water molecule, activated by histidine 57 acting as a base (similarly to its role in the acylation step), attacks the carbonyl carbon of the ester linkage. This attack leads to the formation of a second tetrahedral intermediate.
Collapse of the Second Tetrahedral Intermediate
This second tetrahedral intermediate is also unstable and quickly collapses, releasing the second peptide fragment. A proton is transferred back to Serine 195, regenerating the active site to its original state, ready for another catalytic cycle.
Step 5: Product Release and Enzyme Regeneration
With both peptide fragments released, the enzyme is regenerated to its original form, ready to accept another substrate molecule. The entire catalytic cycle is highly efficient, with the enzyme facilitating the cleavage of peptide bonds at a significantly enhanced rate compared to the uncatalyzed reaction.
The Power of the Catalytic Triad: A Synergistic Partnership
The catalytic triad of Serine 195, Histidine 57, and Aspartate 102 works synergistically. Each residue plays a crucial, interdependent role in facilitating the reaction. The precise positioning of these residues within the active site is critical for the efficiency of the catalytic mechanism. Any alterations in the positions or identities of these amino acids would severely compromise the catalytic activity of the enzyme. The unique arrangement and interplay of these three amino acids represent a remarkable evolutionary achievement.
Beyond the Basics: Further Considerations
The mechanism described above presents a simplified view of chymotrypsin's catalytic process. Several additional factors contribute to its efficiency:
-
Transition State Stabilization: Chymotrypsin's active site is not merely a passive binding site; it actively stabilizes the transition states of the reaction, lowering the activation energy and accelerating the reaction rate. This stabilization is achieved through specific interactions between the enzyme and the substrate during the formation and collapse of the tetrahedral intermediates.
-
Ground State Destabilization: The enzyme's active site also destabilizes the ground state of the substrate, making it more reactive and therefore more susceptible to nucleophilic attack by Serine 195. This destabilization contributes to the overall rate enhancement.
-
Conformational Changes: Subtle conformational changes within the enzyme's structure play a role in substrate binding, catalysis, and product release. These changes are often crucial for efficient substrate access to the active site and for optimizing the interactions between the enzyme and its substrates.
-
Hydrophobic Interactions: The hydrophobic nature of the active site and specificity pocket plays a crucial role in substrate binding and orientation. These interactions contribute to the enzyme's impressive specificity and efficiency.
Chymotrypsin's Significance: A Model Enzyme
Chymotrypsin's mechanism serves as a paradigm for understanding serine protease catalysis. Its relatively well-understood mechanism has provided crucial insights into the principles of enzyme catalysis in general. The studies of chymotrypsin have significantly advanced our understanding of how enzymes achieve such remarkable rate enhancements and specificity in biological systems. Its detailed mechanism continues to inspire advancements in areas such as protein engineering and drug design. The lessons learned from chymotrypsin's elegant catalytic strategy are invaluable for exploring and manipulating enzyme function for various biotechnological applications.
Understanding the mechanism of chymotrypsin catalysis is not just an academic exercise; it has significant implications for various fields. From developing new drugs that target specific proteases to designing improved biocatalysts for industrial applications, the detailed understanding of this enzyme's function remains incredibly valuable. Further research continues to uncover the intricate details of chymotrypsin's catalytic machinery, constantly refining our understanding of this remarkable enzyme. The elegance and efficiency of chymotrypsin's mechanism continue to fascinate and inspire scientists across various disciplines.
Latest Posts
Latest Posts
-
When The Wash Sale Rules Apply The Realized Loss Is
Mar 19, 2025
-
Fixed Costs Expressed On A Per Unit Basis
Mar 19, 2025
-
A Companys Strategic Plan Consists Of
Mar 19, 2025
-
Once The Estimated Depreciation Expense For An Asset Is Calculated
Mar 19, 2025
-
During Lewins Changing Stage Managers Should
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about Put These Steps In The Mechanism Of Chymotrypsin Catalysis . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
