Methylene Blue Chromophore Positive Or Neg
Holbox
Mar 25, 2025 · 5 min read
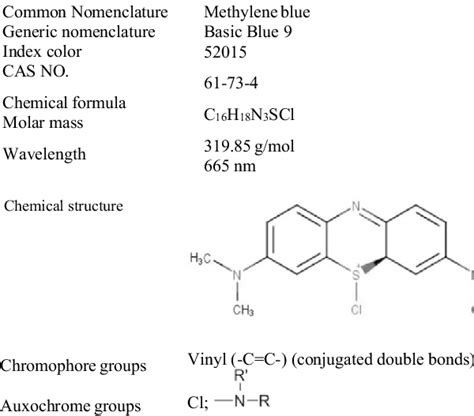
Table of Contents
- Methylene Blue Chromophore Positive Or Neg
- Table of Contents
- Methylene Blue Chromophore: Positive or Negative? Understanding its Role in Staining and Beyond
- Understanding the Methylene Blue Chromophore
- The Role of the Positive Charge
- Interactions with Biological Tissues
- Methylene Blue's Use Beyond Staining: Exploring its Diverse Applications
- Methylene Blue in Medicine:
- Methylene Blue in other Fields:
- The "Positive" and "Negative" Aspects of Methylene Blue’s Chromophore
- Conclusion: Context is Key
- Latest Posts
- Latest Posts
- Related Post
Methylene Blue Chromophore: Positive or Negative? Understanding its Role in Staining and Beyond
Methylene blue, a thiazine dye with the chemical name 3,7-bis(dimethylamino)phenothiazin-5-ium chloride, is widely used as a staining agent in various scientific fields. Its vibrant blue color stems from its chromophore, the part of the molecule responsible for its color and absorption of light. But is this chromophore considered "positive" or "negative"? The answer is nuanced and depends on the context. This article will delve into the properties of methylene blue's chromophore, its interactions with different materials, and the implications of its staining properties in diverse applications.
Understanding the Methylene Blue Chromophore
The chromophore in methylene blue is a conjugated system of alternating single and double bonds within the phenothiazine ring structure. This conjugated system allows for the delocalization of electrons, enabling the absorption of light in the visible spectrum, resulting in its characteristic blue color. The specific wavelength of light absorbed is dependent on the structure and electron distribution within the chromophore. This interaction with light is fundamental to methylene blue's use as a staining agent.
The Role of the Positive Charge
Crucially, methylene blue carries a positive charge. This positive charge is localized on the sulfur atom within the phenothiazine ring. This positive charge plays a significant role in its interaction with biological and other materials. It's this positive charge that drives its interaction with negatively charged components, a key mechanism in its staining capabilities.
Interactions with Biological Tissues
The positive charge of the methylene blue chromophore is central to its staining properties in biological applications. Many components of biological tissues, such as nucleic acids (DNA and RNA) and certain proteins, carry negative charges. The electrostatic attraction between the positively charged methylene blue and these negatively charged components leads to the binding of the dye to the tissues. This is why methylene blue is a valuable tool for staining various biological structures, making them visible under a microscope.
Specific Examples of Methylene Blue Staining:
-
Nuclear staining: Methylene blue stains the nuclei of cells blue due to the interaction between the positively charged dye and the negatively charged DNA within the nucleus. This allows for clear visualization of cell nuclei and their morphology.
-
Connective tissue staining: Methylene blue can stain connective tissues such as collagen and elastin, though not as specifically as other stains. The staining is based on the interaction with the negatively charged components within these tissues.
-
Microbial staining: Methylene blue is used in microbiology as a simple stain to visualize bacteria. The dye binds to the negatively charged components on the bacterial cell surface, allowing for their visualization under a microscope. This is particularly useful for identifying bacterial morphology and size.
Methylene Blue's Use Beyond Staining: Exploring its Diverse Applications
The applications of methylene blue extend far beyond simple staining. Its unique properties, arising from its chromophore and its positive charge, have led to its use in diverse fields:
Methylene Blue in Medicine:
-
Antiseptic and disinfectant: Its antiseptic and disinfectant properties are due to its ability to interact with and inactivate certain microorganisms. It's been used traditionally for treating minor wounds and skin infections.
-
Treatment of methemoglobinemia: Methylene blue is an effective treatment for methemoglobinemia, a condition where a significant portion of hemoglobin is in the oxidized methemoglobin form, impairing oxygen transport. Methylene blue facilitates the reduction of methemoglobin back to its functional form.
-
Diagnostic tool: It's used in some diagnostic procedures, such as visualizing the urinary tract during cystoscopy.
Methylene Blue in other Fields:
-
Photography: While less common now, it has historical significance in photography as a sensitizer for certain photographic processes.
-
Environmental monitoring: Its properties make it useful in certain environmental applications, though its use in this field is less widespread than in biological and medical applications.
The "Positive" and "Negative" Aspects of Methylene Blue’s Chromophore
The term "positive" or "negative" when referring to methylene blue's chromophore is context-dependent and doesn't imply inherent goodness or badness. However, we can analyze its aspects in a way that aligns with this terminology:
"Positive" aspects:
-
Effective Staining: The chromophore’s positive charge facilitates effective staining of negatively charged biological structures, enabling visualization and analysis in microscopy. This is undeniably a highly positive attribute in many research and diagnostic settings.
-
Therapeutic Uses: Its medicinal applications, particularly in the treatment of methemoglobinemia, highlight a significant positive impact on human health.
"Negative" aspects (or potential limitations):
-
Non-specificity: While useful for general staining, its non-specific binding can sometimes lead to background staining or overlapping signals, potentially complicating interpretation. Specific stains are often preferred for more precise identification of cellular structures.
-
Toxicity: Like any chemical substance, methylene blue can exhibit toxicity at high concentrations. Appropriate safety measures and cautious handling are essential when working with this compound.
-
Phototoxicity: Methylene blue can be phototoxic, meaning it can cause damage to cells when exposed to light. This is a crucial consideration in its applications and requires careful management of light exposure during its use.
Conclusion: Context is Key
The characterization of methylene blue's chromophore as "positive" or "negative" is not straightforward. Its positive charge, a defining feature of its chromophore, enables its valuable staining properties and certain therapeutic applications. However, its non-specificity and potential toxicity must also be considered. Ultimately, the usefulness and implications of methylene blue's chromophore are heavily context-dependent and need to be carefully evaluated within the specific application. Its versatility and long history of use attest to its importance in diverse scientific and medical fields, but safe handling and responsible application are paramount. Future research continues to explore the potential of methylene blue, refining its uses and mitigating its limitations. The ongoing investigation of this fascinating dye underscores its enduring relevance in many areas of science and medicine.
Latest Posts
Latest Posts
-
Sb 1577 Exempts A Business Entity From Licensure
Mar 27, 2025
-
Art Labeling Activity Anatomy And Histology Of The Adrenal Gland
Mar 27, 2025
-
Received Customer Purchase Order No 37225
Mar 27, 2025
-
The Net Reaction Catalyzed By Malate Dehydrogenase Is
Mar 27, 2025
-
Evaluate Each Of The Following Expressions Given That
Mar 27, 2025
Related Post
Thank you for visiting our website which covers about Methylene Blue Chromophore Positive Or Neg . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
