The Net Reaction Catalyzed By Malate Dehydrogenase Is
Holbox
Mar 27, 2025 · 5 min read
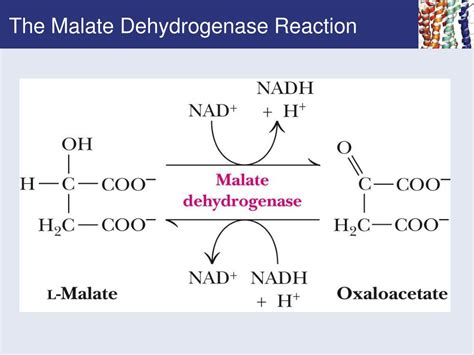
Table of Contents
- The Net Reaction Catalyzed By Malate Dehydrogenase Is
- Table of Contents
- The Net Reaction Catalyzed by Malate Dehydrogenase: A Deep Dive
- The Core Reaction: Oxidizing Malate, Reducing NAD+
- Understanding the Equilibrium: Le Chatelier's Principle in Action
- MDH's Crucial Roles in Cellular Metabolism
- 1. The Citric Acid Cycle (TCA Cycle): A Central Player
- 2. The Malate-Aspartate Shuttle: Bridging Mitochondrial and Cytosolic Compartments
- 3. Other Metabolic Pathways: A Supporting Role
- The Enzyme's Structure and Mechanism: A Closer Look
- Substrate Binding and Orientation: A Precise Arrangement
- Catalytic Mechanism: A Step-by-Step Process
- Regulation of MDH Activity: Maintaining Metabolic Balance
- Isozymes and Tissue-Specific Expression: Tailoring to Metabolic Needs
- Clinical Significance: MDH in Disease and Diagnostics
- Conclusion: An Indispensable Enzyme for Life
- Latest Posts
- Latest Posts
- Related Post
The Net Reaction Catalyzed by Malate Dehydrogenase: A Deep Dive
Malate dehydrogenase (MDH) is a crucial enzyme found in virtually all living organisms. Its role in central metabolic pathways, specifically the citric acid cycle (TCA cycle) and the malate-aspartate shuttle, makes it an essential component for cellular energy production and redox balance. Understanding the net reaction catalyzed by MDH, its mechanism, regulation, and significance in various biological contexts is vital for comprehending fundamental cellular processes. This comprehensive article will delve into these aspects, providing a detailed overview of this important enzyme.
The Core Reaction: Oxidizing Malate, Reducing NAD+
At its heart, the net reaction catalyzed by malate dehydrogenase is the reversible interconversion of malate and oxaloacetate, coupled with the reduction of NAD+ to NADH + H+ or vice versa. This seemingly simple reaction is deceptively complex, playing a pivotal role in several metabolic pathways.
The specific reaction can be written as follows:
Malate + NAD+ ⇌ Oxaloacetate + NADH + H+
This equilibrium demonstrates the enzyme's ability to function in both directions depending on the cellular environment and the prevailing metabolic needs. The direction of the reaction is heavily influenced by the concentrations of the substrates and products, as well as the cellular redox potential.
Understanding the Equilibrium: Le Chatelier's Principle in Action
Le Chatelier's principle dictates that a system at equilibrium will shift to counteract any stress applied to it. In the context of MDH, this means that:
- High concentrations of malate and NAD+ will drive the reaction towards the formation of oxaloacetate and NADH.
- High concentrations of oxaloacetate and NADH will drive the reaction towards the formation of malate and NAD+.
This reversible nature is crucial for the enzyme's diverse roles in cellular metabolism.
MDH's Crucial Roles in Cellular Metabolism
MDH's involvement extends beyond a simple redox reaction. Its participation in key metabolic processes highlights its importance:
1. The Citric Acid Cycle (TCA Cycle): A Central Player
In the TCA cycle, MDH catalyzes the oxidation of malate to oxaloacetate, generating NADH, a critical electron carrier in the electron transport chain (ETC). This NADH subsequently contributes to ATP production via oxidative phosphorylation. This step represents a crucial link between the TCA cycle and energy production. The reaction is part of the final stages of the cycle, providing a key link to the replenishment of oxaloacetate which continues the cycle.
2. The Malate-Aspartate Shuttle: Bridging Mitochondrial and Cytosolic Compartments
The malate-aspartate shuttle is a crucial mechanism for transferring reducing equivalents (NADH) from the cytosol, where glycolysis takes place, into the mitochondria, the powerhouse of the cell. This shuttle involves MDH isoforms located in both the mitochondrial matrix and the cytosol. Cytosolic MDH reduces oxaloacetate to malate using cytosolic NADH, and mitochondrial MDH then oxidizes malate to oxaloacetate, generating mitochondrial NADH for use in the ETC. This process is vital for efficient energy production, especially in tissues with high energy demands.
3. Other Metabolic Pathways: A Supporting Role
While its central roles are in the TCA cycle and malate-aspartate shuttle, MDH also plays a supporting role in other metabolic processes. It contributes to pathways involving amino acid metabolism, gluconeogenesis (glucose synthesis), and the metabolism of various other metabolites.
The Enzyme's Structure and Mechanism: A Closer Look
MDH is a dimeric enzyme, meaning it consists of two identical subunits. Each subunit possesses a distinct active site where the enzymatic reaction takes place. The active site contains crucial amino acid residues involved in substrate binding and catalysis. Understanding the structure is key to appreciating how it facilitates the reaction:
Substrate Binding and Orientation: A Precise Arrangement
The active site of MDH is specifically designed to bind malate and NAD+ in a precise orientation, favoring the interaction between the appropriate atoms for the reaction to proceed efficiently. This precision minimizes the activation energy, allowing the reaction to occur at a physiologically relevant rate.
Catalytic Mechanism: A Step-by-Step Process
The catalytic mechanism of MDH involves several key steps:
- Substrate Binding: Malate and NAD+ bind to the active site.
- Hydride Transfer: A hydride ion (H-) is transferred from the hydroxyl group of malate to the nicotinamide ring of NAD+, reducing NAD+ to NADH.
- Oxidation: The malate molecule undergoes oxidation, becoming oxaloacetate.
- Product Release: The oxaloacetate and NADH molecules are released from the active site.
Regulation of MDH Activity: Maintaining Metabolic Balance
MDH activity is tightly regulated to ensure the proper functioning of metabolic pathways. Regulation can occur through several mechanisms:
- Substrate Availability: The concentrations of malate, oxaloacetate, NAD+, and NADH directly influence the direction and rate of the reaction.
- Allosteric Regulation: Although not as extensively studied as in some other enzymes, MDH activity can be influenced by allosteric effectors, molecules that bind to the enzyme at sites other than the active site and modulate its activity.
- Post-translational Modifications: Covalent modifications, such as phosphorylation, can alter the enzyme's activity.
Isozymes and Tissue-Specific Expression: Tailoring to Metabolic Needs
MDH exists as several isozymes, which are different forms of the same enzyme with subtle variations in their amino acid sequences. These isozymes are often expressed in a tissue-specific manner, reflecting the differing metabolic demands of various tissues. For example, the heart may express different isoforms than the liver, optimizing enzyme function based on metabolic requirements.
Clinical Significance: MDH in Disease and Diagnostics
MDH's widespread involvement in cellular metabolism makes it relevant in various clinical contexts:
- Biomarkers of Disease: Elevated levels of MDH in blood serum can be indicative of certain diseases, particularly those involving tissue damage, such as myocardial infarction (heart attack), liver damage, and certain cancers. The presence of elevated MDH levels serves as an indicator of cell damage and is helpful in diagnosing such conditions.
- Therapeutic Targets: Given its central role in metabolism, MDH is a potential target for drug development in various diseases, particularly those involving metabolic dysfunction.
Conclusion: An Indispensable Enzyme for Life
Malate dehydrogenase, with its crucial role in the TCA cycle, the malate-aspartate shuttle, and other metabolic pathways, is an indispensable enzyme for life. Its reversible catalysis of the malate-oxaloacetate interconversion is a fundamental process in cellular energy production and redox balance. Understanding the enzyme's structure, mechanism, regulation, and clinical significance provides valuable insights into fundamental cellular processes and potential therapeutic strategies for various diseases. Further research continues to unravel the nuances of MDH's function and its role in maintaining cellular homeostasis. The ongoing exploration of this enzyme promises to yield even more significant discoveries in the fields of biochemistry and medicine.
Latest Posts
Latest Posts
-
The Three Regions On A Pressure Enthalpy Chart Are
Mar 31, 2025
-
Does Oligopoly Describe Rolex Better Than Monopolistic Competition
Mar 31, 2025
-
Norton Field Guide To Writing 6th Edition
Mar 31, 2025
-
One Concern Voiced By Critics Of Globalization Is That
Mar 31, 2025
-
Draw Both The Organic And Inorganic Intermediate Species
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about The Net Reaction Catalyzed By Malate Dehydrogenase Is . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
