Match The Type Of Bond With Its Description.
Holbox
Mar 20, 2025 · 6 min read
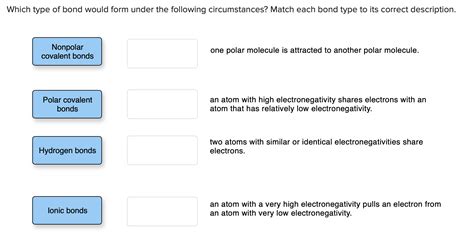
Table of Contents
Match the Type of Bond with its Description: A Comprehensive Guide
Understanding chemical bonds is fundamental to grasping the properties and behaviors of matter. This comprehensive guide will delve into the various types of chemical bonds, providing detailed descriptions and examples to help you confidently match each bond type with its characteristics. We'll explore the nuances of each bond, focusing on the forces of attraction and the resulting properties of the bonded atoms or molecules.
The Fundamental Forces: Ionic, Covalent, and Metallic Bonds
Chemical bonds are essentially the forces that hold atoms together to form molecules and compounds. These forces arise from the electrostatic attraction between oppositely charged particles. The three primary types of chemical bonds are:
- Ionic Bonds: Formed through the electrostatic attraction between oppositely charged ions. This involves the complete transfer of electrons from one atom (typically a metal) to another (typically a nonmetal).
- Covalent Bonds: Formed by the sharing of electrons between atoms. This typically occurs between nonmetal atoms.
- Metallic Bonds: Occur in metals and are characterized by a "sea" of delocalized electrons shared among a lattice of positively charged metal ions.
Delving Deeper: Ionic Bonds
What are Ionic Bonds?
Ionic bonds are formed when one atom completely transfers one or more electrons to another atom. This transfer creates ions: positively charged cations (atoms that have lost electrons) and negatively charged anions (atoms that have gained electrons). The strong electrostatic attraction between these oppositely charged ions constitutes the ionic bond.
Characteristics of Ionic Compounds:
- High melting and boiling points: The strong electrostatic forces require significant energy to overcome.
- Crystalline structure: Ions arrange themselves in a regular, repeating three-dimensional lattice structure.
- Brittle: Disruption of the crystal lattice can lead to repulsion between like charges, causing fracturing.
- Conduct electricity when molten or dissolved in water: Free-moving ions are necessary for electrical conductivity.
- Often soluble in polar solvents: Polar solvents like water can interact with the charged ions, disrupting the ionic lattice.
Examples of Ionic Compounds:
- Sodium chloride (NaCl): Sodium (Na) loses one electron to become Na⁺, and chlorine (Cl) gains one electron to become Cl⁻. The electrostatic attraction between Na⁺ and Cl⁻ forms the ionic bond.
- Magnesium oxide (MgO): Magnesium (Mg) loses two electrons to become Mg²⁺, and oxygen (O) gains two electrons to become O²⁻.
- Potassium iodide (KI): Potassium (K) loses one electron to become K⁺, and iodine (I) gains one electron to become I⁻.
Covalent Bonds: Sharing is Caring
Understanding Covalent Bonds
In covalent bonds, atoms share electrons to achieve a stable electron configuration, typically resembling a noble gas. This sharing creates a region of high electron density between the atoms, resulting in a strong attractive force.
Types of Covalent Bonds:
- Single bonds: Involve the sharing of one pair of electrons. Represented by a single line (-) in structural formulas.
- Double bonds: Involve the sharing of two pairs of electrons. Represented by a double line (=).
- Triple bonds: Involve the sharing of three pairs of electrons. Represented by a triple line (≡).
Polarity in Covalent Bonds:
The electronegativity difference between the atoms involved determines the polarity of a covalent bond.
- Nonpolar covalent bonds: Occur when the electronegativity difference is negligible or very small (typically less than 0.5). Electrons are shared relatively equally. Example: Cl₂.
- Polar covalent bonds: Occur when there's a significant electronegativity difference (typically between 0.5 and 1.7). Electrons are shared unequally, resulting in a partial positive charge (δ+) on the less electronegative atom and a partial negative charge (δ-) on the more electronegative atom. Example: H₂O.
Characteristics of Covalent Compounds:
- Lower melting and boiling points than ionic compounds: Covalent bonds are generally weaker than ionic bonds.
- Can be solids, liquids, or gases at room temperature: Depending on the strength of the intermolecular forces.
- Generally poor conductors of electricity: Lack of free-moving charged particles.
- Often soluble in nonpolar solvents: Similar solubility characteristics due to similar intermolecular forces.
Examples of Covalent Compounds:
- Water (H₂O): Oxygen shares electrons with two hydrogen atoms.
- Methane (CH₄): Carbon shares electrons with four hydrogen atoms.
- Carbon dioxide (CO₂): Carbon forms double bonds with two oxygen atoms.
Metallic Bonds: A Sea of Electrons
The Nature of Metallic Bonds
Metallic bonds are unique and occur in metals. The valence electrons of metal atoms are delocalized, meaning they are not associated with a specific atom but rather move freely throughout the metal lattice. This "sea" of delocalized electrons creates strong attractive forces holding the positively charged metal ions together.
Characteristics of Metallic Compounds:
- High melting and boiling points: Strong metallic bonds require considerable energy to break.
- Malleable and ductile: The delocalized electrons allow metal atoms to slide past each other without disrupting the metallic bond.
- Good conductors of electricity and heat: The free-moving electrons facilitate the transfer of both electricity and heat.
- Lustrous: The delocalized electrons interact with light, giving metals their characteristic shine.
Examples of Metals exhibiting Metallic Bonding:
- Iron (Fe)
- Copper (Cu)
- Gold (Au)
- Aluminum (Al)
Beyond the Basics: Hydrogen Bonds and Van der Waals Forces
While ionic, covalent, and metallic bonds are the primary types, other intermolecular forces play significant roles in the properties of substances.
Hydrogen Bonds: A Special Case
Hydrogen bonds are a type of strong dipole-dipole interaction. They occur between a hydrogen atom bonded to a highly electronegative atom (like oxygen, nitrogen, or fluorine) and another electronegative atom in a different molecule. Hydrogen bonds are crucial for the properties of water and many biological molecules.
Van der Waals Forces: Weak but Important
Van der Waals forces are weak intermolecular forces that arise from temporary fluctuations in electron distribution around atoms and molecules. These forces are collectively weak but can become significant in large molecules or when many such interactions occur. Types of Van der Waals forces include London dispersion forces, dipole-dipole forces, and ion-dipole forces.
Matching Bonds with Descriptions: A Practice Exercise
To solidify your understanding, let's test your knowledge with a matching exercise. Match the following bond types with their descriptions:
Bond Types:
- Ionic Bond
- Covalent Bond
- Metallic Bond
- Hydrogen Bond
- Van der Waals Forces
Descriptions:
A. Involves the sharing of electrons between atoms. B. Occurs in metals, characterized by a sea of delocalized electrons. C. Formed by the electrostatic attraction between oppositely charged ions. D. A strong dipole-dipole interaction involving hydrogen bonded to a highly electronegative atom. E. Weak intermolecular forces arising from temporary fluctuations in electron distribution.
Answers:
- C
- A
- B
- D
- E
Conclusion: Understanding the Bonds that Hold the World Together
Understanding the different types of chemical bonds is paramount to comprehending the vast diversity of substances in the world around us. From the solid crystals of salt to the flowing liquid of water and the malleable nature of metals, the type of bond dictates the unique properties of each material. This comprehensive guide has provided you with the tools to confidently identify and differentiate between these fundamental forces of attraction, paving the way for a deeper understanding of chemistry and its impact on our world. By understanding these fundamental concepts, you can better predict the properties of materials and their behavior in various contexts, opening up a world of possibilities for scientific exploration and innovation.
Latest Posts
Latest Posts
-
The Use Of Money Contributes To Economic Efficiency Because
Mar 20, 2025
-
A Favorable Cost Variance Occurs When
Mar 20, 2025
-
What Is The Likely Product Of The Reaction Shown
Mar 20, 2025
-
Which One Of The Following Is A Strong Acid
Mar 20, 2025
-
Shiraz Needs To Record Receipt Of A Vendor Invoice
Mar 20, 2025
Related Post
Thank you for visiting our website which covers about Match The Type Of Bond With Its Description. . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
