What Is The Likely Product Of The Reaction Shown
Holbox
Mar 20, 2025 · 6 min read
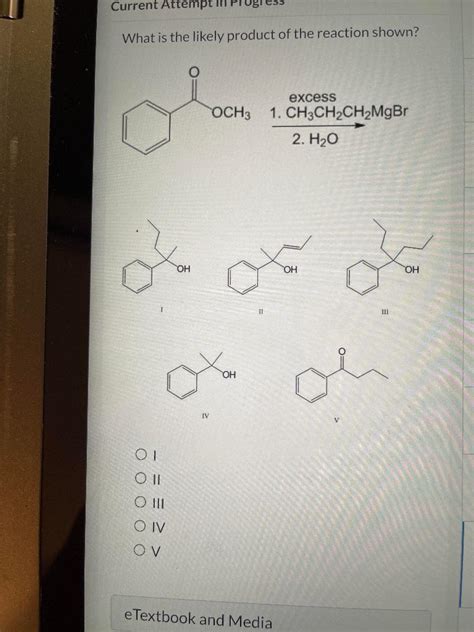
Table of Contents
What is the Likely Product of the Reaction Shown? A Deep Dive into Predicting Reaction Outcomes
Predicting the outcome of a chemical reaction is a cornerstone of chemistry. While memorizing countless reactions is impractical, understanding fundamental principles allows us to make educated guesses about the likely products. This article delves into the process of predicting reaction products, focusing on various reaction types and the factors influencing their outcomes. We'll explore strategies for analyzing reaction schemes and determining the most probable products formed. This will involve examining reaction mechanisms, understanding reaction kinetics, and considering thermodynamic factors.
Understanding Reaction Types: The Foundation of Prediction
Before predicting products, classifying the reaction type is crucial. Several common reaction types serve as building blocks for more complex reactions:
1. Acid-Base Reactions (Neutralization):
These reactions involve the transfer of a proton (H⁺) from an acid to a base. The products are typically salt and water.
Example: HCl (aq) + NaOH (aq) → NaCl (aq) + H₂O (l)
Prediction Strategy: Identify the acid and base. The cation from the base and the anion from the acid combine to form the salt. Water is always a product.
2. Precipitation Reactions:
These reactions occur when two aqueous solutions containing soluble salts are mixed, resulting in the formation of an insoluble solid (precipitate). Solubility rules are essential for predicting precipitates.
Example: AgNO₃ (aq) + NaCl (aq) → AgCl (s) + NaNO₃ (aq)
Prediction Strategy: Consult a solubility table. If a combination of cations and anions forms an insoluble compound, a precipitate will form.
3. Redox Reactions (Oxidation-Reduction):
These reactions involve the transfer of electrons. One species is oxidized (loses electrons), and another is reduced (gains electrons). Identifying oxidation states is vital for predicting products.
Example: 2Fe (s) + 3Cl₂ (g) → 2FeCl₃ (s)
Prediction Strategy: Determine the oxidation states of reactants. The species that loses electrons is oxidized, and the species that gains electrons is reduced. The products will reflect the new oxidation states. Balancing redox reactions often requires half-reaction methods.
4. Combustion Reactions:
These reactions involve the rapid reaction of a substance with oxygen, usually producing heat and light. Hydrocarbons often combust to produce carbon dioxide and water.
Example: CH₄ (g) + 2O₂ (g) → CO₂ (g) + 2H₂O (l)
Prediction Strategy: For hydrocarbons, the products are usually carbon dioxide and water. For other substances, the products depend on the reactants and the conditions of the reaction.
5. Single Displacement Reactions:
These reactions involve one element replacing another in a compound. Activity series are crucial for predicting whether a reaction will occur.
Example: Zn (s) + CuSO₄ (aq) → ZnSO₄ (aq) + Cu (s)
Prediction Strategy: Refer to the activity series. A more reactive metal will displace a less reactive metal from its compound.
6. Double Displacement Reactions:
These reactions involve the exchange of ions between two compounds. Solubility rules are important for predicting the products.
Example: BaCl₂ (aq) + Na₂SO₄ (aq) → BaSO₄ (s) + 2NaCl (aq)
Prediction Strategy: Exchange the cations and anions between the two reactants. Check solubility rules to determine if a precipitate will form.
Beyond Basic Reaction Types: Factors Influencing Product Formation
While classifying the reaction type provides a framework, several additional factors influence the final product distribution:
1. Reaction Conditions: Temperature and Pressure
Temperature and pressure significantly impact reaction outcomes. Higher temperatures generally increase reaction rates, but may also favor different products. Pressure primarily affects reactions involving gases.
Example: The Haber-Bosch process for ammonia synthesis (N₂ + 3H₂ ⇌ 2NH₃) is highly pressure-dependent, favoring ammonia formation at high pressures.
2. Catalysts: Altering Reaction Pathways
Catalysts speed up reactions by providing an alternative reaction pathway with lower activation energy. They can influence product selectivity, favoring the formation of specific products.
Example: The use of a zeolite catalyst in cracking reactions alters the product distribution, increasing the yield of gasoline-range hydrocarbons.
3. Concentration of Reactants: Equilibrium Considerations
The concentration of reactants impacts the position of equilibrium in reversible reactions. Higher reactant concentrations shift the equilibrium towards product formation.
Example: In the esterification reaction between an acid and an alcohol, using excess alcohol shifts the equilibrium towards ester formation, increasing yield.
4. Solvent Effects: Polarity and Solvation
The solvent plays a crucial role by influencing the solvation of reactants and intermediates. Polar solvents favor polar reactions, while nonpolar solvents favor nonpolar reactions.
Example: Grignard reactions, which are highly sensitive to moisture, require anhydrous (water-free) solvents.
5. Steric Hindrance: Spatial Effects
Steric hindrance, the interference caused by bulky groups in a molecule, can influence reaction rates and product selectivity. Bulky groups can hinder the approach of reactants, leading to different products or slower reaction rates.
Example: In SN1 reactions, steric hindrance around the carbocation intermediate can influence the product distribution.
Advanced Techniques for Product Prediction: Mechanism and Kinetics
For more complex reactions, a deeper understanding of the reaction mechanism and kinetics is crucial for accurate product prediction.
1. Reaction Mechanisms: Step-by-Step Analysis
Reaction mechanisms provide a detailed step-by-step description of how a reaction proceeds. Analyzing the mechanism reveals intermediate species and transition states, crucial for predicting products.
Example: The SN1 and SN2 mechanisms for nucleophilic substitution reactions lead to different products depending on the substrate and nucleophile.
2. Reaction Kinetics: Rate Laws and Rate-Determining Steps
Reaction kinetics studies the rate of reaction. Identifying the rate-determining step allows us to understand which steps control the overall reaction rate and influence product distribution.
Example: In consecutive reactions, the rate-determining step determines the overall reaction rate and influences the concentrations of intermediates and final products.
3. Thermodynamic Considerations: Gibbs Free Energy
Thermodynamics predicts whether a reaction is spontaneous (favorable) based on the change in Gibbs free energy (ΔG). A negative ΔG indicates a spontaneous reaction, while a positive ΔG indicates a non-spontaneous reaction.
Example: The formation of water from hydrogen and oxygen is thermodynamically favorable (ΔG < 0), indicating a spontaneous reaction under standard conditions.
Putting it All Together: A Practical Approach to Product Prediction
Predicting reaction products is not a purely theoretical exercise. A systematic approach combines theoretical knowledge with practical considerations:
- Identify the reaction type: Classify the reaction as acid-base, redox, precipitation, etc.
- Analyze the reactants: Determine the structure and properties of each reactant.
- Consider the reaction conditions: Note the temperature, pressure, solvent, and presence of any catalysts.
- Apply relevant principles: Use solubility rules, activity series, oxidation state changes, and other relevant concepts.
- Predict the products: Based on the above, predict the likely products.
- Assess the reaction mechanism: For complex reactions, delve into the mechanism to understand the step-by-step process.
- Consider thermodynamic factors: Determine the spontaneity of the reaction using Gibbs free energy.
- Evaluate the results: Review the predicted products and consider potential side reactions or competing pathways.
Conclusion: A Continuous Learning Process
Predicting the likely product of a chemical reaction is a complex process requiring a strong foundation in chemical principles. While this article provides a comprehensive overview, it’s important to remember that predicting reaction outcomes is a skill developed through continuous practice and a deep understanding of reaction mechanisms, kinetics, and thermodynamics. The more experience you gain in analyzing different reactions and their associated factors, the better your ability to predict their outcomes accurately. Remember that even with a thorough understanding, unexpected results can sometimes occur, highlighting the dynamic and fascinating nature of chemistry.
Latest Posts
Latest Posts
-
An Account Is Said To Have A Debit Balance If
Mar 21, 2025
-
Classify The Radicals Into The Appropriate Categories
Mar 21, 2025
-
Two Spacecraft Are Following Paths In Space Given By
Mar 21, 2025
-
When Consumers Decide To Purchase A Particular Product They
Mar 21, 2025
-
Refer To Figure 4 17 At A Price Of
Mar 21, 2025
Related Post
Thank you for visiting our website which covers about What Is The Likely Product Of The Reaction Shown . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
