Classify The Radicals Into The Appropriate Categories
Holbox
Mar 19, 2025 · 7 min read
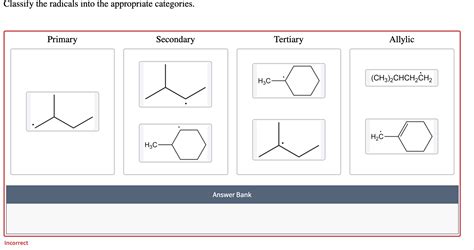
Table of Contents
Classifying Radicals: A Comprehensive Guide
Understanding radicals, those fundamental building blocks of chemical compounds, is crucial for mastering chemistry. This comprehensive guide delves deep into the classification of radicals, exploring their various categories based on structure, stability, and reactivity. We'll examine different types, providing examples and insights into their behavior in chemical reactions. By the end, you'll possess a robust understanding of radical classification, empowering you to predict their behavior and understand their significance in various chemical processes.
What are Radicals?
Before diving into classification, let's establish a firm understanding of what radicals are. Radicals, also known as free radicals, are atoms, molecules, or ions that possess an unpaired electron in their outermost electron shell. This unpaired electron makes them highly reactive, readily seeking another electron to pair with and achieve a more stable state. This inherent instability drives their participation in numerous chemical reactions.
The presence of this unpaired electron is often represented by a single dot (•) next to the chemical symbol or formula. For instance, a methyl radical is represented as •CH₃. This dot signifies the unpaired electron, highlighting the radical's reactive nature.
Classifying Radicals: Key Categories
Several methods exist for classifying radicals, depending on the criteria used. We'll focus on the most common and useful classifications:
1. Classification by Structure:
This approach categorizes radicals based on the nature of the atom or group carrying the unpaired electron. Some prominent subcategories include:
-
Alkyl Radicals: These radicals contain an unpaired electron on a carbon atom that's part of an alkyl group (a saturated hydrocarbon chain). Examples include methyl (•CH₃), ethyl (•CH₂CH₃), and propyl (•CH₂CH₂CH₃) radicals. Alkyl radicals are relatively stable compared to other types but are still highly reactive. Their stability increases with the size of the alkyl group due to hyperconjugation, a phenomenon where electron density from neighboring C-H bonds stabilizes the unpaired electron.
-
Aryl Radicals: These feature an unpaired electron on a carbon atom within an aromatic ring system, such as a benzene ring. Phenyl radical (•C₆H₅) is a prime example. Aryl radicals exhibit unique properties due to the resonance stabilization provided by the delocalized π electrons of the aromatic ring. This resonance significantly increases their stability compared to alkyl radicals.
-
Vinyl Radicals: Characterized by an unpaired electron residing on a carbon atom that’s double-bonded to another carbon atom (in a vinyl group, -CH=CH₂). Vinyl radicals are less stable than alkyl radicals due to the sp² hybridization of the carbon atom bearing the unpaired electron. The p orbital holding the unpaired electron is perpendicular to the plane of the molecule and does not participate effectively in hyperconjugation, leading to less stability.
-
Allyl Radicals: These radicals possess an unpaired electron on a carbon atom adjacent to a double bond (-CH₂-CH=CH₂). Allyl radicals demonstrate enhanced stability due to resonance stabilization from the adjacent double bond. The unpaired electron can delocalize across the entire allyl system, creating two resonance structures. This resonance significantly increases stability, making them more reactive than vinyl radicals but less so than aryl radicals.
-
Benzyl Radicals: Similar to allyl radicals, benzyl radicals exhibit resonance stabilization. The unpaired electron resides on a carbon atom directly attached to a benzene ring. The electron can delocalize into the aromatic ring, contributing to significant stability.
2. Classification by Stability:
Radical stability dictates their reactivity and lifespan. More stable radicals react more slowly and have longer lifetimes. The stability primarily depends on the electron-donating or electron-withdrawing groups attached to the carbon atom with the unpaired electron.
-
Tertiary Radicals (3°): These radicals have the unpaired electron on a carbon atom bonded to three other carbon atoms. They are the most stable due to the hyperconjugative effect of three alkyl groups, which donate electron density to stabilize the unpaired electron.
-
Secondary Radicals (2°): These radicals have the unpaired electron on a carbon atom bonded to two other carbon atoms. They are less stable than tertiary radicals but more stable than primary radicals.
-
Primary Radicals (1°): These have the unpaired electron on a carbon atom bonded to only one other carbon atom. They are less stable than secondary and tertiary radicals due to fewer electron-donating alkyl groups.
-
Methyl Radicals (•CH₃): These are primary radicals but are relatively more stable than other primary radicals due to the symmetry of the molecule.
3. Classification by Reactivity:
Radical reactivity is strongly linked to their stability. Highly unstable radicals are extremely reactive, readily participating in reactions to achieve stability. Conversely, more stable radicals react more slowly.
-
Highly Reactive Radicals: These are typically less stable radicals like primary and vinyl radicals. They readily participate in chain reactions, readily abstracting hydrogen atoms or adding to unsaturated bonds.
-
Moderately Reactive Radicals: Secondary and allyl radicals fall into this category. Their reactivity is intermediate compared to highly and less reactive radicals.
-
Less Reactive Radicals: Tertiary, aryl, and benzyl radicals possess greater stability due to resonance or steric factors, exhibiting lower reactivity than other radicals.
4. Classification by Source:
Radicals can be classified based on how they are formed:
-
Photochemically Generated Radicals: These radicals are formed through the absorption of light energy, causing bond cleavage and the formation of radicals. A classic example is the photolysis of diatomic halogen molecules (e.g., Cl₂) to produce chlorine radicals (•Cl).
-
Thermally Generated Radicals: These radicals are formed through the application of heat, promoting homolytic cleavage of bonds. Heat-induced decomposition of peroxides is a common method of thermal radical generation.
-
Radicals from Redox Reactions: Some redox reactions, involving electron transfer, can lead to the formation of radicals. Reactions involving metal ions with variable oxidation states can often produce radicals as intermediates or products.
Factors Affecting Radical Stability:
Several factors significantly influence radical stability:
-
Hyperconjugation: The interaction between the unpaired electron and the sigma (σ) bonding electrons of adjacent C-H bonds. This interaction stabilizes the radical by delocalizing the unpaired electron. The more alkyl groups attached to the carbon atom with the unpaired electron, the greater the hyperconjugation and, therefore, the greater the stability.
-
Resonance: The delocalization of the unpaired electron across a conjugated system, such as in allyl, benzyl, and aryl radicals. This delocalization reduces the electron density at any single atom, increasing stability.
-
Inductive Effect: The electron-donating or electron-withdrawing properties of substituents attached to the carbon atom with the unpaired electron. Electron-donating groups stabilize radicals, while electron-withdrawing groups destabilize them.
-
Steric Effects: Bulky groups surrounding the radical center can influence stability by hindering the approach of reactants or stabilizing the radical through steric shielding.
Importance of Radical Classification:
Understanding the classification of radicals is crucial for several reasons:
-
Predicting Reactivity: Knowing the type and stability of a radical allows chemists to predict its reactivity and participation in chemical reactions.
-
Designing Reactions: Chemists utilize this knowledge to design and control reactions involving radicals, synthesizing specific products.
-
Understanding Reaction Mechanisms: Radical classification helps elucidate the mechanisms of numerous chemical reactions, including polymerization, combustion, and atmospheric chemistry.
-
Developing New Materials: Radicals play a vital role in the synthesis of various materials. Understanding their properties facilitates the development of new materials with desired characteristics.
-
Biological Significance: Radicals, particularly those produced within biological systems, can have harmful effects (oxidative stress). Understanding their behavior is crucial in understanding diseases and developing treatments.
Conclusion:
This in-depth exploration of radical classification provides a robust foundation for understanding the behavior and properties of these highly reactive species. By categorizing radicals based on structure, stability, reactivity, and source, we can gain valuable insight into their participation in various chemical and biological processes. This knowledge is fundamental for chemists and researchers involved in various fields, including organic chemistry, materials science, and biochemistry. Further exploration into specific radical reactions and their applications will solidify this understanding and empower deeper comprehension of chemical processes. Remember, the more you understand the nuances of radical chemistry, the better equipped you will be to interpret, predict, and control chemical reactions effectively.
Latest Posts
Latest Posts
-
The Control Devices Used In Pneumatics Are Called
Mar 19, 2025
-
Social Conventional Reasoning Asserts That Conventional Rules Are
Mar 19, 2025
-
Which Of The Following Is True Of Business Rules
Mar 19, 2025
-
Industrial Machinery Is An Example Of
Mar 19, 2025
-
Two Systems Of Defensive Driving Are
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about Classify The Radicals Into The Appropriate Categories . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
