Which One Of The Following Is A Strong Acid
Holbox
Mar 20, 2025 · 5 min read
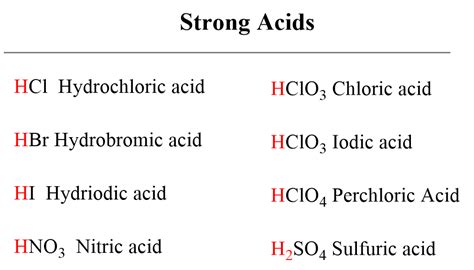
Table of Contents
Which One of the Following is a Strong Acid? Understanding Acid Strength and Dissociation
Determining whether an acid is strong or weak is crucial in various fields, from chemistry and environmental science to medicine and industrial processes. This article delves deep into the concept of acid strength, focusing on how to identify strong acids and differentiating them from their weaker counterparts. We'll explore the underlying principles governing acid dissociation, provide a comprehensive list of common strong acids, and offer practical examples to solidify your understanding.
Understanding Acid Strength: A Deeper Dive
The strength of an acid is determined by its ability to donate a proton (H⁺) to a base. A strong acid is one that completely dissociates (ionizes) in water, meaning it releases all its protons into the solution. In contrast, a weak acid only partially dissociates, meaning only a small fraction of its molecules donate protons. This difference significantly impacts the acidity (pH) of a solution.
The Dissociation Constant (Ka)
The extent of dissociation is quantified using the acid dissociation constant (Ka). A higher Ka value indicates a stronger acid because it signifies a greater tendency to donate protons. Strong acids have very large Ka values (typically greater than 1), while weak acids have small Ka values (much less than 1). For practical purposes, when dealing with strong acids, we often consider them to completely dissociate, simplifying calculations.
Factors Affecting Acid Strength
Several factors influence an acid's strength:
-
Bond Polarity: A highly polar bond between the hydrogen atom and the rest of the molecule makes it easier for the proton to be released, thus increasing acid strength. The more electronegative the atom bonded to the hydrogen, the stronger the acid.
-
Bond Strength: Weaker bonds are more easily broken, leading to easier proton donation and thus, a stronger acid.
-
Size and Electronegativity of the Anion: The stability of the conjugate base (the anion formed after proton donation) plays a significant role. Larger and more electronegative anions are more stable, favoring dissociation and hence, a stronger acid.
-
Resonance Effects: If the conjugate base can delocalize the negative charge through resonance, it becomes more stable, leading to a stronger acid.
Common Strong Acids: A Comprehensive List
While numerous acids exist, only a select few qualify as strong acids. Memorizing this list is crucial for any chemistry student or professional:
-
Hydrochloric acid (HCl): Found in stomach acid and used in various industrial processes. Its complete dissociation in water makes it a highly corrosive substance.
-
Hydrobromic acid (HBr): Similar in properties to HCl, it's also a highly corrosive strong acid.
-
Hydroiodic acid (HI): Another highly corrosive strong acid that completely dissociates in water.
-
Nitric acid (HNO₃): A powerful oxidizing agent widely used in the production of fertilizers and explosives. Its complete dissociation contributes to its corrosive nature.
-
Sulfuric acid (H₂SO₄): A diprotic acid (donates two protons), it's a very strong acid in its first dissociation step. While the second dissociation is weaker, it's still considered a strong acid overall due to the complete dissociation of the first proton. It’s crucial in various industrial applications, including the production of fertilizers and petroleum refining.
-
Perchloric acid (HClO₄): Considered one of the strongest acids, its complete dissociation and high reactivity make it a powerful oxidizing agent. However, its use is limited due to safety concerns.
Important Note: The strength of an acid is not necessarily related to its concentration. A dilute solution of a strong acid is still a strong acid; it just contains fewer acid molecules. Conversely, a concentrated solution of a weak acid remains a weak acid, although it may have a lower pH due to the higher concentration of undissociated molecules.
Differentiating Strong and Weak Acids: Practical Examples
Let's illustrate the difference between strong and weak acids with some practical examples:
Example 1: Comparing HCl and Acetic Acid (CH₃COOH)
Hydrochloric acid (HCl) is a strong acid; it completely dissociates in water:
HCl(aq) → H⁺(aq) + Cl⁻(aq)
Acetic acid (CH₃COOH), on the other hand, is a weak acid; it only partially dissociates:
CH₃COOH(aq) ⇌ H⁺(aq) + CH₃COO⁻(aq)
The double arrow (⇌) indicates an equilibrium where both the reactants and products coexist. Only a small fraction of acetic acid molecules donate protons.
Example 2: The pH Scale and Acid Strength
The pH scale is a logarithmic measure of hydrogen ion concentration. Strong acids have very low pH values (typically below 1), indicating a high concentration of H⁺ ions. Weak acids have higher pH values, closer to neutral (pH 7).
Example 3: Conductivity
Strong acids are excellent conductors of electricity due to the high concentration of ions in their solutions. Weak acids are poor conductors because they have a much lower concentration of ions.
Applications of Strong Acids
The unique properties of strong acids make them indispensable in various applications:
-
Industrial Processes: Sulfuric acid is a cornerstone of many industrial processes, including fertilizer production, petroleum refining, and metal processing. Hydrochloric acid is used in cleaning, etching, and pickling metals.
-
Chemical Synthesis: Strong acids are often catalysts in organic chemical reactions. They facilitate many crucial synthetic pathways in the pharmaceutical and materials science industries.
-
Analytical Chemistry: Strong acids are essential in titrations and other analytical techniques to determine the concentration of unknown solutions.
-
Medicine: Hydrochloric acid is a component of stomach acid, crucial for digestion.
Safety Precautions When Handling Strong Acids
Working with strong acids necessitates strict adherence to safety protocols:
-
Personal Protective Equipment (PPE): Always wear appropriate PPE, including safety goggles, gloves, and lab coats, when handling strong acids.
-
Ventilation: Ensure adequate ventilation to minimize exposure to acid fumes.
-
Dilution: Always add acid to water, never water to acid, to avoid dangerous exothermic reactions.
-
Neutralization: In case of spills, use appropriate neutralizing agents, such as sodium bicarbonate (baking soda), under careful supervision.
-
Proper Disposal: Dispose of strong acids according to local regulations and safety guidelines.
Conclusion: Understanding the Power of Strong Acids
Strong acids, with their complete dissociation in water and high reactivity, play a crucial role in diverse scientific and industrial fields. Understanding their properties, differentiating them from weak acids, and adhering to safety precautions are vital for anyone working with these powerful chemicals. This article has provided a comprehensive overview, equipping you with the knowledge to identify strong acids, understand their behavior, and appreciate their significance in various applications. Remember, responsible handling and thorough understanding of safety protocols are paramount when dealing with these potent substances.
Latest Posts
Latest Posts
-
A Responsibility Accounting Performance Report Displays
Mar 21, 2025
-
Both Learned Helplessness And Depression Are Marked By
Mar 21, 2025
-
Histamine Causes All The Following Except
Mar 21, 2025
-
Cash Flow To Stockholders Is Defined As
Mar 21, 2025
-
Final Step To Installing A Floating Vinyl Floor
Mar 21, 2025
Related Post
Thank you for visiting our website which covers about Which One Of The Following Is A Strong Acid . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
