Lysosomes Are Membrane-bound Vesicles That Arise From
Holbox
Mar 27, 2025 · 7 min read
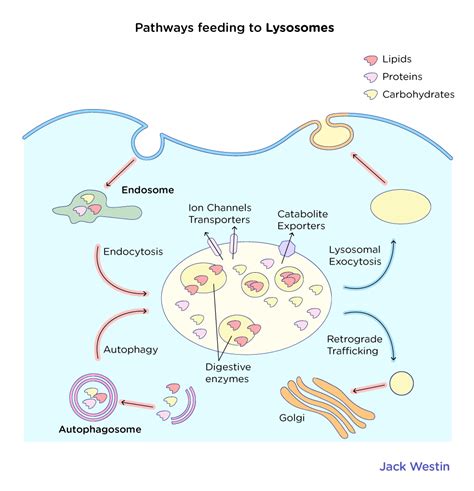
Table of Contents
- Lysosomes Are Membrane-bound Vesicles That Arise From
- Table of Contents
- Lysosomes: Membrane-Bound Vesicles That Arise From the Trans-Golgi Network – A Deep Dive
- 1. The Origin Story: From Trans-Golgi Network to Mature Lysosome
- 2. The Diverse Roles of Lysosomes: More Than Just Waste Disposal
- 3. Lysosomal Storage Disorders: When the Recycling System Fails
- 4. Lysosomes and Human Health: Beyond Storage Disorders
- 5. Future Directions in Lysosome Research
- Latest Posts
- Latest Posts
- Related Post
Lysosomes: Membrane-Bound Vesicles That Arise From the Trans-Golgi Network – A Deep Dive
Lysosomes are vital organelles found within animal cells, acting as the cell's recycling and waste disposal system. Often described as the cell's "stomach," these membrane-bound vesicles are responsible for breaking down various cellular components and external materials through a process known as autophagy and phagocytosis. Understanding their origin, function, and implications in various diseases is crucial to comprehending cellular biology and human health.
This in-depth article will explore the fascinating world of lysosomes, starting with their origins and delving into their intricate roles in maintaining cellular homeostasis.
1. The Origin Story: From Trans-Golgi Network to Mature Lysosome
Contrary to some simplified depictions, lysosomes don't spontaneously appear in the cell. They undergo a complex biogenesis process, primarily originating from the trans-Golgi network (TGN). The TGN is the final compartment of the Golgi apparatus, a crucial organelle responsible for modifying, sorting, and packaging proteins and lipids for various destinations within and outside the cell.
The journey of a lysosome begins with the budding of vesicles from the TGN. These vesicles contain a unique set of hydrolytic enzymes, collectively known as acid hydrolases. These enzymes are remarkably diverse, capable of breaking down a wide range of macromolecules including proteins, lipids, carbohydrates, and nucleic acids. The acidic environment within the lysosome (pH ~4.5-5.0), maintained by a proton pump embedded in the lysosomal membrane, is crucial for the optimal activity of these enzymes. This acidic environment is essential because it protects the cell from the potentially harmful effects of these enzymes should they leak into the cytoplasm.
The newly formed vesicles are not yet fully mature lysosomes. They undergo further maturation and fusion with other vesicles, including endosomes (compartments that receive materials from endocytosis) and autophagosomes (structures involved in autophagy). This fusion process is crucial for delivering the substrates for the acid hydrolases to degrade. The fusion of these vesicles with each other and with pre-existing lysosomes results in a complex and dynamic network within the cell, constantly adapting to the cell's needs.
Specific molecular markers and sorting signals play pivotal roles in directing the lysosomal enzymes to their destination. Mannose-6-phosphate (M6P) is a critical marker, added to acid hydrolases within the Golgi apparatus. M6P receptors in the TGN recognize this marker, facilitating the packaging of acid hydrolases into vesicles destined for lysosomes. Defects in this targeting mechanism can lead to lysosomal storage disorders, as we'll discuss later.
2. The Diverse Roles of Lysosomes: More Than Just Waste Disposal
Lysosomes are far more than mere waste disposal units; they play multifaceted roles in maintaining cellular health and function. Their actions are crucial for:
-
Autophagy: This critical cellular process involves the degradation of damaged organelles, misfolded proteins, and other cellular debris. Autophagosomes, double-membrane vesicles that engulf cellular components, fuse with lysosomes, delivering their contents for degradation. This process is crucial for maintaining cellular quality control and removing potentially harmful substances. Dysregulation of autophagy is linked to various diseases, including cancer and neurodegenerative disorders.
-
Phagocytosis: This process involves the engulfment of larger extracellular particles, such as bacteria or cellular debris, by specialized cells such as macrophages. The ingested material is enclosed within a phagosome, which then fuses with a lysosome, where the contents are broken down. This is a crucial part of the innate immune response, protecting the organism from invading pathogens.
-
Endocytosis: This process involves the uptake of smaller molecules and fluids from the extracellular environment. Endosomes, formed during endocytosis, fuse with lysosomes to degrade the ingested material. This is important for nutrient uptake and recycling of cellular components.
-
Nutrient Recycling: Lysosomes break down macromolecules into their constituent monomers (amino acids, fatty acids, sugars, and nucleotides). These monomers are then transported back into the cytoplasm to be reused in various metabolic pathways, illustrating the remarkable efficiency of the lysosomal system in conserving cellular resources.
-
Signal Transduction: Emerging research suggests that lysosomes also play a role in cellular signaling pathways. Lysosomal membrane proteins can interact with other cellular components, influencing various cellular processes.
3. Lysosomal Storage Disorders: When the Recycling System Fails
The intricate nature of lysosomal function makes them susceptible to dysfunction. Lysosomal storage disorders (LSDs) are a group of inherited metabolic diseases resulting from defects in lysosomal enzymes or proteins involved in lysosomal function. Because of these defects, the substrates that should be broken down accumulate within the lysosomes, leading to a progressive build-up of undigested materials.
These accumulated substances can disrupt cellular function and cause a range of symptoms, depending on the specific enzyme deficiency and the affected tissues. The severity of these disorders varies widely. Some LSDs are relatively mild, while others can be fatal. LSDs can impact virtually any organ system and manifest with a diverse range of clinical presentations, including neurological problems, skeletal abnormalities, cardiac dysfunction, and impaired vision.
Examples of LSDs include:
-
Tay-Sachs disease: Deficiency of the enzyme β-hexosaminidase A, leading to the accumulation of gangliosides in the brain.
-
Gaucher disease: Deficiency of the enzyme β-glucocerebrosidase, leading to the accumulation of glucocerebroside in various tissues.
-
Hunter syndrome: Deficiency of iduronate-2-sulfatase, leading to the accumulation of glycosaminoglycans.
-
Pompe disease: Deficiency of acid α-glucosidase, leading to glycogen accumulation.
The diagnosis of LSDs often involves biochemical assays to measure enzyme activity and genetic testing to identify the underlying genetic defect. Treatment options vary depending on the specific disorder, but may include enzyme replacement therapy, substrate reduction therapy, or bone marrow transplantation. Research continues to explore novel therapeutic strategies, including gene therapy, to treat these debilitating diseases.
4. Lysosomes and Human Health: Beyond Storage Disorders
Lysosomal dysfunction is implicated in a much broader range of human health issues than just LSDs. Research increasingly highlights the involvement of lysosomes in:
-
Cancer: Lysosomes play a role in cancer cell survival and metastasis. Altered lysosomal function can affect autophagy, impacting tumor growth and progression.
-
Neurodegenerative diseases: Dysregulation of lysosomal function contributes to the accumulation of misfolded proteins and damaged organelles, contributing to the pathogenesis of diseases like Alzheimer's and Parkinson's.
-
Infectious diseases: Lysosomes are crucial for the intracellular destruction of pathogens. Impaired lysosomal function can increase susceptibility to infections.
-
Aging: Age-related decline in lysosomal function is believed to contribute to various age-related pathologies.
Further research into lysosomal biology is crucial to developing novel therapies for these and other diseases. A deeper understanding of the mechanisms regulating lysosomal function, the intricate interactions between lysosomes and other cellular components, and the implications of lysosomal dysfunction in various disease states is vital for progress in medicine.
5. Future Directions in Lysosome Research
The field of lysosomal biology is undergoing a period of rapid expansion. Advanced techniques, including high-resolution microscopy, proteomics, and genomics, are providing increasingly detailed insights into the intricate workings of lysosomes and their interactions with other cellular compartments.
Key areas of ongoing research include:
-
Developing novel therapeutic strategies for LSDs and other lysosome-related diseases. This includes advancements in gene therapy, enzyme replacement therapy, and the development of small molecule inhibitors targeting specific pathways involved in lysosomal dysfunction.
-
Investigating the role of lysosomes in various diseases, including cancer, neurodegenerative disorders, and aging. A better understanding of the mechanisms linking lysosomal dysfunction to these diseases will pave the way for developing effective therapeutic interventions.
-
Exploring the potential of lysosomes as therapeutic targets. Modulating lysosomal function could offer new avenues for treating a wide range of diseases.
-
Unraveling the complexity of lysosomal signaling and its impact on cellular processes. Lysosomes are not merely passive waste disposal units but actively participate in cellular signaling and regulation. Further understanding of these roles will provide new insights into cellular biology and disease mechanisms.
In conclusion, lysosomes are far more complex and multifaceted than their initial description as cellular "waste disposal units" might suggest. Their pivotal role in maintaining cellular homeostasis and their implication in a broad spectrum of diseases highlight their importance in the context of both fundamental biology and human health. Continued research into lysosomal biology promises to yield valuable discoveries, leading to improved diagnostics, novel therapies, and a deeper understanding of human health and disease. The journey of these remarkable organelles, from their origin in the trans-Golgi network to their multifaceted roles in maintaining cellular health, remains a captivating and crucial area of scientific investigation.
Latest Posts
Latest Posts
-
One Concern Voiced By Critics Of Globalization Is That
Mar 31, 2025
-
Draw Both The Organic And Inorganic Intermediate Species
Mar 31, 2025
-
Estimate The Following Limit Using Graphs Or Tables
Mar 31, 2025
-
Solve For Simplify Your Answer As Much As Possible
Mar 31, 2025
-
Match The Description With The Correct Type Of Neuron
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about Lysosomes Are Membrane-bound Vesicles That Arise From . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
