In Each Reaction Box Place The Best Reagent And Conditions
Holbox
Mar 21, 2025 · 6 min read
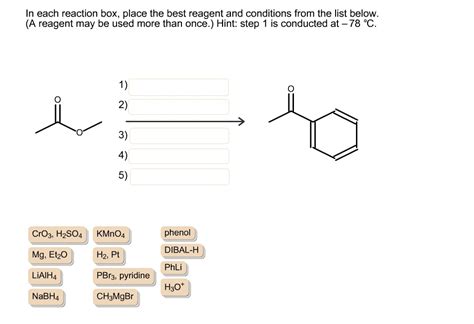
Table of Contents
- In Each Reaction Box Place The Best Reagent And Conditions
- Table of Contents
- In Each Reaction Box: Placing the Best Reagent and Conditions for Organic Synthesis
- Understanding the Fundamentals: Reaction Mechanisms and Selectivity
- Case Studies: Choosing the Best Reagent and Conditions
- 1. Alkyl Halide Synthesis: SN2 vs. SN1
- 2. Alkene Formation: E1 vs. E2
- 3. Grignard Reactions: Forming Carbon-Carbon Bonds
- 4. Oxidation Reactions: Varying Reagents for Selective Oxidation
- 5. Reduction Reactions: Different Reducing Agents for Different Functional Groups
- The Importance of Solvent Selection
- Conclusion: Strategic Reagent and Condition Selection
- Latest Posts
- Latest Posts
- Related Post
In Each Reaction Box: Placing the Best Reagent and Conditions for Organic Synthesis
Organic chemistry, at its core, is a dance of molecular transformations. Mastering this dance requires a deep understanding of reaction mechanisms and a keen eye for selecting the optimal reagents and conditions to achieve the desired transformation efficiently and selectively. Choosing the best reagent and conditions isn't just about getting the product; it's about maximizing yield, minimizing side reactions, and employing environmentally friendly methods whenever possible. This article will delve into the crucial aspects of reagent and condition selection, providing examples to illustrate the principles involved.
Understanding the Fundamentals: Reaction Mechanisms and Selectivity
Before diving into specific examples, it's essential to grasp the underlying principles that govern reagent and condition selection. Every organic reaction proceeds through a specific mechanism, involving a series of elementary steps. Understanding this mechanism is crucial for predicting the outcome of a reaction and choosing the appropriate reagents and conditions.
Key factors to consider:
-
Reaction Mechanism: Is the reaction an SN1, SN2, E1, E2, addition, elimination, or a more complex multi-step process? Each mechanism has its own requirements regarding reagents and conditions.
-
Substrate Structure: The structure of the starting material significantly influences the reaction pathway and the selectivity of the reaction. Steric hindrance, electronic effects, and the presence of functional groups all play a role.
-
Reagent Reactivity: Different reagents possess varying reactivities. Choosing a reagent that is too reactive can lead to unwanted side reactions, while a reagent that is too unreactive may fail to produce the desired product.
-
Reaction Conditions: Temperature, solvent, and pressure all influence reaction rate and selectivity. Careful control of these parameters is often essential for success.
-
Selectivity: The ability to favor one product over others is crucial. This involves choosing reagents and conditions that selectively target a specific functional group or a particular stereochemical outcome.
Case Studies: Choosing the Best Reagent and Conditions
Let's examine several common organic transformations, highlighting the choices involved in reagent and condition selection:
1. Alkyl Halide Synthesis: SN2 vs. SN1
The conversion of alcohols to alkyl halides is a frequently encountered transformation. The choice between SN1 and SN2 conditions depends heavily on the structure of the alcohol.
a) SN2 Reaction (Primary Alcohols):
For primary alcohols, the SN2 mechanism is favored. This generally involves using a strong nucleophile like chloride ion (Cl⁻), bromide ion (Br⁻), or iodide ion (I⁻), and a strong acid like HBr or HCl. The reaction is typically carried out at a relatively low temperature to avoid elimination side reactions. For example, conversion of a primary alcohol to an alkyl bromide can effectively utilize concentrated HBr at room temperature.
b) SN1 Reaction (Tertiary Alcohols):
Tertiary alcohols readily undergo SN1 reactions. Here, the alcohol is first protonated by a strong acid (H₂SO₄ or HCl), generating a carbocation intermediate. This is then attacked by a nucleophile (e.g., chloride ion, bromide ion). The reaction often occurs at elevated temperatures and doesn't require a strong nucleophile.
2. Alkene Formation: E1 vs. E2
The synthesis of alkenes from alkyl halides or alcohols hinges on the elimination reactions: E1 and E2.
a) E2 Reaction (Strong Base):
The E2 mechanism requires a strong base like potassium tert-butoxide (t-BuOK) or sodium ethoxide (NaOEt). These bases abstract a proton from a β-carbon, leading to simultaneous bond breaking and formation, resulting in alkene formation. The reaction is generally favored for secondary and primary alkyl halides. The choice of base can influence regioselectivity (Zaitsev's rule vs. Hofmann elimination).
b) E1 Reaction (Weak Base, Tertiary Substrate):
E1 reactions often proceed with weaker bases or in the presence of strong acids. They are favored for tertiary substrates because they involve the formation of a stable tertiary carbocation intermediate. A common approach involves heating the tertiary alkyl halide in a polar protic solvent.
3. Grignard Reactions: Forming Carbon-Carbon Bonds
Grignard reagents (RMgX) are powerful tools for forming carbon-carbon bonds. They react readily with carbonyl compounds (aldehydes, ketones, esters, etc.) to form new carbon-carbon bonds.
The reaction typically occurs in anhydrous ether solvents (like diethyl ether or THF) to prevent the decomposition of the Grignard reagent by water. After the addition of the Grignard reagent to the carbonyl compound, an acidic workup (e.g., aqueous HCl) is required to protonate the alkoxide intermediate and generate the alcohol product. The success of Grignard reactions relies heavily on maintaining anhydrous conditions and choosing an appropriate solvent and temperature.
4. Oxidation Reactions: Varying Reagents for Selective Oxidation
Oxidation reactions are crucial for converting alcohols to aldehydes, ketones, or carboxylic acids. The choice of oxidizing agent dictates the level of oxidation.
a) PCC (Pyridinium Chlorochromate): A mild oxidizing agent that selectively converts primary alcohols to aldehydes and secondary alcohols to ketones.
b) Jones Reagent (CrO₃/H₂SO₄): A stronger oxidizing agent that can oxidize primary alcohols to carboxylic acids and secondary alcohols to ketones.
c) Swern Oxidation: Uses DMSO and oxalyl chloride, a powerful method for oxidizing primary and secondary alcohols, especially sensitive substrates.
5. Reduction Reactions: Different Reducing Agents for Different Functional Groups
Reduction reactions transform functional groups into less oxidized forms. Various reducing agents are employed, each with its own reactivity and selectivity.
a) Lithium Aluminum Hydride (LiAlH₄): A powerful reducing agent that reduces a wide range of functional groups, including ketones, aldehydes, esters, and carboxylic acids to alcohols.
b) Sodium Borohydride (NaBH₄): A milder reducing agent that selectively reduces aldehydes and ketones to alcohols.
c) Catalytic Hydrogenation: Utilizes hydrogen gas (H₂) and a metal catalyst (e.g., Pd/C, Pt/C) to reduce alkenes and alkynes to alkanes.
The Importance of Solvent Selection
The choice of solvent is often overlooked but is crucial for reaction success. The solvent affects the rate of reaction, selectivity, and solubility of reagents and products. Consider these aspects:
-
Polar Protic Solvents: Solvents like water, methanol, and ethanol are suitable for reactions involving polar reactants and intermediates. They can also participate in hydrogen bonding.
-
Polar Aprotic Solvents: Solvents like acetone, DMF, and DMSO are good choices when strong nucleophiles are required because they don't inhibit nucleophilic reactivity through hydrogen bonding.
-
Nonpolar Solvents: Solvents like hexane, diethyl ether, and toluene are suitable for reactions involving nonpolar reactants.
Conclusion: Strategic Reagent and Condition Selection
Selecting the best reagent and conditions for any organic reaction requires a deep understanding of reaction mechanisms, substrate structure, reagent reactivity, and the influence of reaction parameters. Careful consideration of these factors is essential for achieving high yields, excellent selectivity, and environmentally benign synthetic routes. This detailed exploration of various reaction types and reagent choices serves as a foundation for developing a strategic and efficient approach to organic synthesis. Continual learning, practice, and a problem-solving mindset will refine your ability to navigate the complexities of organic chemistry, allowing you to confidently choose the best reagents and conditions for your specific reaction needs. By mastering this crucial skill, you’ll enhance your ability to synthesize complex molecules with precision and efficiency.
Latest Posts
Latest Posts
-
What Is Unusual About Glutathiones Structure
Mar 28, 2025
-
A Significant Weakness Of The Ordinal Scale Is
Mar 28, 2025
-
Two Important Advantages Of Secondary Data Are That They Are
Mar 28, 2025
-
Providing Services And Receiving Cash Will
Mar 28, 2025
-
How To Find Maturity Risk Premium
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about In Each Reaction Box Place The Best Reagent And Conditions . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
