How Many Valence Electrons Does Br Have
Holbox
Mar 16, 2025 · 5 min read
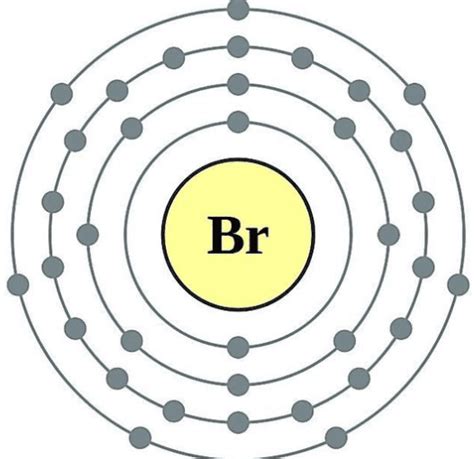
Table of Contents
How Many Valence Electrons Does Br Have? A Deep Dive into Bromine's Electronic Structure
Bromine (Br), a fascinating halogen element, plays a crucial role in various chemical reactions and industrial processes. Understanding its electronic structure, particularly the number of valence electrons, is fundamental to comprehending its chemical behavior and reactivity. This comprehensive guide delves deep into the question: how many valence electrons does Br have? We'll explore the concept of valence electrons, examine bromine's electronic configuration, and discuss the implications of its valence electrons on its chemical properties.
Understanding Valence Electrons: The Key to Reactivity
Before we pinpoint the number of valence electrons in bromine, let's establish a clear understanding of what valence electrons are. Valence electrons are the electrons located in the outermost shell (or energy level) of an atom. These electrons are the most loosely held and are directly involved in chemical bonding. They determine an atom's reactivity, its ability to form chemical bonds with other atoms, and the types of bonds it can form (ionic, covalent, metallic). The number of valence electrons significantly influences an element's chemical properties and its position within the periodic table.
Atoms strive to achieve a stable electron configuration, often resembling that of a noble gas. This principle, known as the octet rule, dictates that atoms tend to gain, lose, or share electrons to achieve eight electrons in their outermost shell (exceptions exist, particularly for elements beyond the second period). Understanding this drive for stability is crucial for predicting chemical behavior.
Determining Bromine's Valence Electrons: Electronic Configuration
Bromine's atomic number is 35, meaning it has 35 protons and 35 electrons in a neutral atom. To determine the number of valence electrons, we need to examine its electronic configuration. The electronic configuration describes how electrons are distributed among different energy levels and subshells within an atom.
Bromine's electronic configuration is: 1s²2s²2p⁶3s²3p⁶4s²3d¹⁰4p⁵.
Let's break this down:
-
1s², 2s², 2p⁶, 3s², 3p⁶: These represent the inner shells, completely filled with electrons. These electrons are tightly bound to the nucleus and do not participate in chemical bonding. They are considered core electrons.
-
4s²3d¹⁰4p⁵: This represents the outermost shell, which contains the valence electrons. Notice that the 4s subshell is filled before the 3d subshell, even though the 3d subshell has a lower principal quantum number (n=3). This is due to the energy levels of the subshells. The 4s orbital is lower in energy than the 3d orbital.
The crucial part is the 4s²4p⁵. Adding the electrons in the 4s and 4p subshells gives us a total of 7 valence electrons (2 + 5 = 7).
Therefore, bromine (Br) has 7 valence electrons.
Implications of Bromine's Seven Valence Electrons
Having seven valence electrons profoundly impacts bromine's chemical behavior. It's only one electron short of achieving a stable octet, making it highly reactive. To achieve a stable configuration, bromine readily gains one electron, forming a bromide ion (Br⁻) with a -1 charge. This is why bromine readily participates in ionic bonding with metals, forming ionic compounds like sodium bromide (NaBr).
However, bromine also readily forms covalent bonds by sharing electrons with other nonmetals. This is because sharing electrons allows both atoms to achieve a more stable electron configuration. Examples include bromine monochloride (BrCl) and bromine trifluoride (BrF₃). The ability to form both ionic and covalent bonds demonstrates the versatility of bromine's reactivity, determined by its seven valence electrons.
Bromine's Position in the Periodic Table and Valence Electrons
Bromine's position in the periodic table further reinforces our understanding of its valence electrons. Bromine is a halogen, belonging to Group 17 (or VIIA). Elements within the same group share similar chemical properties due to having the same number of valence electrons. All halogens have seven valence electrons, explaining their similar reactivity and tendency to form -1 ions or share one electron to complete their octet.
Exploring Bromine's Compounds and its Valence Electrons
The impact of bromine's seven valence electrons extends to the properties of its numerous compounds. For instance, the ionic nature of many bromine compounds leads to high melting and boiling points due to the strong electrostatic forces between ions. In contrast, covalent bromine compounds exhibit lower melting and boiling points because of weaker intermolecular forces.
The reactivity of bromine compounds is also directly related to the number of valence electrons. Compounds with bromine in a higher oxidation state (meaning it's sharing more electrons) tend to be stronger oxidizing agents, capable of accepting electrons from other substances. This oxidizing power finds applications in various industrial processes, including water purification and bleaching.
Beyond the Octet Rule: Bromine's Expanded Octet
While the octet rule provides a helpful framework, it's important to acknowledge that some elements, including bromine, can exceed the octet. This occurs when bromine forms compounds with highly electronegative elements like fluorine. In compounds such as bromine pentafluoride (BrF₅) and bromine heptafluoride (BrF₇), bromine utilizes its d orbitals to accommodate more than eight electrons in its valence shell, demonstrating the limitations of the octet rule for heavier elements.
Bromine's Role in Biological Systems and Valence Electrons
Bromine, while not as prevalent as other halogens like chlorine and iodine, still plays a role in biological systems. Organic bromine compounds are found in marine organisms, and some bromine compounds possess antimicrobial properties. The reactivity of bromine, directly linked to its seven valence electrons, is crucial for the biological activity of these compounds. While the detailed mechanisms are complex, understanding the fundamental role of valence electrons remains central to comprehending the biological relevance of bromine compounds.
Conclusion: The Significance of Bromine's Seven Valence Electrons
In conclusion, bromine (Br) possesses seven valence electrons, a defining characteristic that governs its chemical behavior and reactivity. This number determines its tendency to form -1 ions or share electrons to achieve a stable octet, influencing the formation of ionic and covalent compounds, its position in the periodic table, and its role in biological systems. Understanding the concept of valence electrons and their significance allows us to predict and interpret the diverse chemical interactions of bromine and its compounds. The unique properties arising from its seven valence electrons make bromine an element with significant industrial and scientific importance. Further exploration into bromine's chemistry requires a firm grasp of its electronic structure and the profound influence of its seven valence electrons.
Latest Posts
Latest Posts
-
What Is The Value Of I
Mar 17, 2025
-
The Accounts In The Ledger Of Monroe Entertainment Co
Mar 17, 2025
-
A Process Cost Accounting System Is Most Appropriate When
Mar 17, 2025
-
An Example Of A Breach Of Ephi Is
Mar 17, 2025
-
What Is Involved In Safety Monitoring
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about How Many Valence Electrons Does Br Have . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
