How Many Different Kinds Of 13c Peaks Will Be Seen
Holbox
Mar 21, 2025 · 5 min read
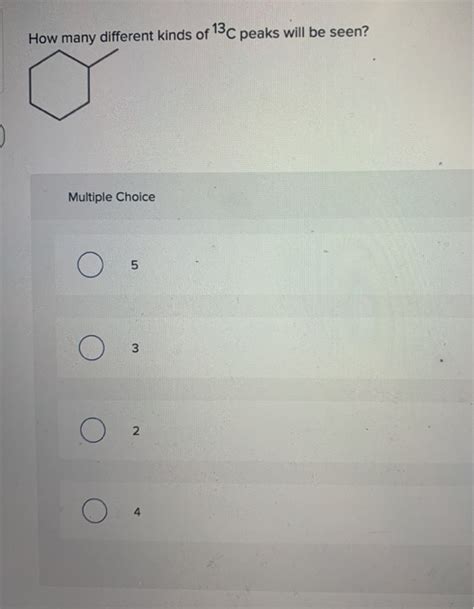
Table of Contents
- How Many Different Kinds Of 13c Peaks Will Be Seen
- Table of Contents
- How Many Different Kinds of 13C Peaks Will Be Seen in NMR Spectroscopy?
- Understanding the Basics of ¹³C NMR Spectroscopy
- Predicting the Number of ¹³C Peaks: Examples and Strategies
- Advanced Considerations
- Conclusion
- Latest Posts
- Latest Posts
- Related Post
How Many Different Kinds of 13C Peaks Will Be Seen in NMR Spectroscopy?
Nuclear Magnetic Resonance (NMR) spectroscopy, particularly Carbon-13 NMR (¹³C NMR), is a powerful technique used to determine the carbon-containing functional groups in organic molecules. Understanding the number of distinct ¹³C peaks observed in a ¹³C NMR spectrum provides crucial information about the molecule's structure and symmetry. However, predicting the exact number of peaks isn't always straightforward and depends on several factors, including the molecule's symmetry, the presence of isotopic isomers, and the NMR spectrometer's capabilities.
This article delves into the intricacies of predicting the number of ¹³C peaks, exploring various scenarios and considering the effects of different molecular features. We'll unravel the relationship between molecular structure and the observed ¹³C NMR spectrum, providing a comprehensive understanding of this vital spectroscopic technique.
Understanding the Basics of ¹³C NMR Spectroscopy
Before diving into the complexities of peak prediction, let's refresh our understanding of the fundamental principles of ¹³C NMR. ¹³C is a naturally occurring isotope of carbon (approximately 1.1% abundance), possessing a nuclear spin (I = ½). When placed in a strong magnetic field, the ¹³C nuclei can absorb radiofrequency radiation at specific frequencies, leading to the generation of a ¹³C NMR spectrum.
The chemical shift, measured in parts per million (ppm), is the key parameter in ¹³C NMR. It represents the resonance frequency of a particular carbon atom relative to a standard reference compound (usually tetramethylsilane, TMS). The chemical shift is highly sensitive to the electronic environment of the carbon atom. Carbon atoms in different chemical environments will experience different magnetic fields, resulting in distinct chemical shifts and therefore separate peaks in the spectrum.
Key Factors influencing the Number of Peaks:
- Chemically Equivalent Carbons: Carbon atoms with identical chemical environments are considered chemically equivalent. They resonate at the same frequency and therefore produce a single peak in the spectrum. Symmetry plays a significant role in determining chemical equivalence.
- Chemical Shift Differences: The resolution of the NMR spectrometer dictates the minimum chemical shift difference required to observe two distinct peaks. Closely spaced peaks might appear as a single, broadened peak if the resolution is insufficient.
- Coupling: ¹³C atoms can couple to other ¹³C atoms (¹³C-¹³C coupling) or to ¹H atoms (¹³C-¹H coupling). However, ¹³C-¹³C coupling is often weak and usually not observed in standard ¹³C NMR experiments due to the low natural abundance of ¹³C. Proton decoupling is routinely employed to simplify the spectrum by removing ¹³C-¹H coupling. This results in simpler spectra with just singlets for each distinct carbon environment.
- Dynamic Processes: In molecules undergoing rapid conformational changes or interconversions (e.g., rotation around single bonds), the observed chemical shifts might represent an average of the different conformations. This can lead to fewer peaks than expected based solely on the static structure.
- Isotopic Effects: The presence of other isotopes, especially deuterium (²H), can influence chemical shifts slightly. However, this effect is often small and is not the primary factor determining the number of peaks.
Predicting the Number of ¹³C Peaks: Examples and Strategies
Predicting the number of distinct ¹³C peaks requires careful consideration of the molecule's structure and symmetry. Let's examine some illustrative examples:
Example 1: Ethane (CH₃CH₃)
Ethane possesses two chemically equivalent methyl groups (CH₃). Therefore, only one ¹³C peak will be observed in the ¹³C NMR spectrum.
Example 2: Ethanol (CH₃CH₂OH)
Ethanol contains three distinct types of carbon atoms: the methyl carbon (CH₃), the methylene carbon (CH₂), and the carbonyl carbon (C=O). Consequently, we expect to see three distinct ¹³C peaks in its spectrum.
Example 3: Benzene (C₆H₆)
Benzene's high symmetry results in all six carbon atoms being chemically equivalent. Therefore, only one ¹³C peak is observed.
Example 4: 1,2-Dichlorobenzene
1,2-Dichlorobenzene has four distinct carbon environments and thus will exhibit four peaks. This is true despite the overall symmetry of the molecule because the carbons immediately adjacent to the chlorines are different from the ones further away.
Example 5: 1,3,5-Trichlorobenzene
The molecule has only two distinct carbons, those bonded to chlorine and those not. Therefore, there will be two peaks in the ¹³C spectrum.
Example 6: More Complex Molecules:
For larger and more complex molecules, predicting the number of peaks can become challenging. It requires a detailed analysis of the molecule's symmetry and the identification of chemically equivalent carbon atoms. Software packages are often employed for larger molecules to aid in predicting chemical shifts and thus the number of peaks. This can become complex with many isomers being possible, further complicating the analysis. Detailed structural analysis using techniques such as DEPT (Distortionless Enhancement by Polarization Transfer) and HSQC (Heteronuclear Single Quantum Correlation) experiments can further help in resolving the assignment of peaks.
Advanced Considerations
Several advanced factors can also influence the number of observable ¹³C peaks:
- Overlapping Peaks: Peaks can sometimes overlap, making it difficult to distinguish them. This often necessitates higher resolution techniques or using advanced NMR processing methods.
- Peak Broadening: Various factors like rapid exchange processes or paramagnetic impurities can lead to peak broadening, making it difficult to resolve closely spaced peaks.
- Sensitivity: The inherent low natural abundance of ¹³C (1.1%) can result in weak signals, particularly for carbons with low relaxation rates, making it challenging to observe all peaks clearly. This can be enhanced through techniques like signal averaging and using pulse sequences that enhance sensitivity.
- Solvent Effects: The solvent used in the NMR experiment can slightly influence chemical shifts, potentially causing small shifts in peak positions. This is typically a minor effect.
Conclusion
Predicting the exact number of ¹³C peaks in a ¹³C NMR spectrum is a critical aspect of structure elucidation in organic chemistry. While chemically equivalent carbons yield a single peak, factors like molecular symmetry, spectrometer resolution, dynamic processes, and overlapping peaks can complicate this prediction. By carefully analyzing the molecule's structure, considering its symmetry elements, and taking into account the various factors discussed, one can make accurate predictions of the number of observable ¹³C NMR peaks. Advanced techniques in NMR spectroscopy further facilitate the assignment of the peaks to specific carbons in the molecule, leading to the complete determination of the structure. Furthermore, the utilization of software packages specializing in NMR prediction can substantially simplify the process, especially for large and complex molecules where manual prediction is impractical. Understanding the nuances of ¹³C NMR spectroscopy is essential for researchers in organic chemistry and related fields.
Latest Posts
Latest Posts
-
Preganglionic Fibers Leave The Cns And Then Synapse On
Mar 28, 2025
-
Portia Grant Is An Employee Who Is Paid Monthly
Mar 28, 2025
-
Art Labeling Activity Basic Anatomy Of The Skin
Mar 28, 2025
-
As Organic Vegetables Increase In Popularity
Mar 28, 2025
-
The Highest Barrier That A Projectile Can Clear Is
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about How Many Different Kinds Of 13c Peaks Will Be Seen . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
