Draw The Major Products Of This Reaction. Ignore Inorganic Byproducts
Holbox
Mar 15, 2025 · 5 min read
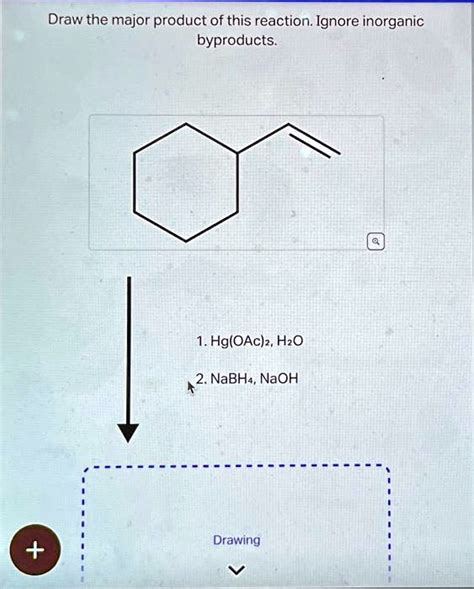
Table of Contents
Drawing the Major Products of Organic Reactions: A Comprehensive Guide
Predicting the major products of organic reactions is a cornerstone of organic chemistry. It requires a deep understanding of reaction mechanisms, functional group transformations, and the principles of regioselectivity and stereoselectivity. This article delves into the process, providing a structured approach to tackling these problems and illustrating the concepts with numerous examples. We'll ignore inorganic byproducts for simplicity and focus on the organic molecules formed.
Understanding Reaction Mechanisms: The Key to Prediction
Before attempting to predict products, a firm grasp of the underlying reaction mechanism is crucial. Mechanisms detail the step-by-step process of bond breaking and bond formation, explaining why specific products are favored. Common reaction mechanisms include:
-
SN1 (Substitution Nucleophilic Unimolecular): This mechanism involves a two-step process: 1) carbocation formation and 2) nucleophilic attack. Carbocation stability dictates the regioselectivity (which carbon atom the nucleophile attacks). More substituted carbocations (tertiary > secondary > primary) are more stable. Racemization is often observed due to planar nature of carbocations.
-
SN2 (Substitution Nucleophilic Bimolecular): This is a concerted, one-step process where nucleophilic attack and leaving group departure occur simultaneously. Steric hindrance plays a major role; primary carbons react fastest, while tertiary carbons are essentially unreactive. SN2 reactions proceed with inversion of configuration.
-
E1 (Elimination Unimolecular): Similar to SN1, E1 reactions involve a two-step process: 1) carbocation formation and 2) base abstraction of a proton. The more substituted alkene (Zaitsev's rule) is generally the major product.
-
E2 (Elimination Bimolecular): This is a concerted, one-step process where base abstraction of a proton and leaving group departure occur simultaneously. The stereochemistry of the starting material influences the stereochemistry of the alkene product (anti-periplanar arrangement preferred). Zaitsev's rule generally applies, favoring the more substituted alkene.
-
Addition Reactions (Electrophilic and Nucleophilic): These reactions involve adding atoms or groups across a multiple bond (e.g., double or triple bond). Regioselectivity and stereosectivity are important considerations. Markovnikov's rule often applies to electrophilic additions to alkenes.
Factors Influencing Product Formation
Several factors influence which product is formed predominantly:
-
Substrate Structure: The structure of the starting material significantly dictates reactivity and product formation. Steric hindrance, presence of electron-withdrawing or electron-donating groups, and the type of functional groups all play crucial roles.
-
Reagent: The nature of the reagent (nucleophile, electrophile, base, etc.) influences both the reaction mechanism and the product. Strong bases favor elimination reactions, while weaker bases may favor substitution. The nucleophile's strength and steric bulk also play significant roles.
-
Reaction Conditions: Temperature, solvent, and concentration can impact the reaction pathway and product distribution. Higher temperatures generally favor elimination reactions. The solvent can influence the rate and selectivity of the reaction.
Illustrative Examples
Let's illustrate product prediction with specific examples.
Example 1: SN1 Reaction
Consider the reaction of 2-bromo-2-methylpropane with methanol.
(CH3)3CBr + CH3OH → ?
This is a classic SN1 reaction. The tertiary carbocation intermediate is readily formed, followed by nucleophilic attack by methanol. The major product is tert-butyl methyl ether ((CH3)3COCH3). Since the carbocation is planar, a racemic mixture will be formed.
Example 2: SN2 Reaction
Consider the reaction of bromomethane with sodium hydroxide.
CH3Br + NaOH → ?
This is an SN2 reaction. The hydroxide ion attacks the carbon atom bearing the bromine, leading to inversion of configuration. The major product is methanol (CH3OH).
Example 3: E1 Reaction
Consider the dehydration of 2-methyl-2-butanol with sulfuric acid.
(CH3)2C(OH)CH2CH3 + H2SO4 → ?
This is an E1 reaction. The carbocation intermediate can lose a proton from either of two carbons, leading to the formation of two alkenes. However, the more substituted alkene (2-methyl-2-butene) is the major product, following Zaitsev's rule.
Example 4: E2 Reaction
Consider the reaction of 2-bromobutane with potassium tert-butoxide.
CH3CHBrCH2CH3 + t-BuOK → ?
This is an E2 reaction. The bulky tert-butoxide base favors the formation of the less substituted alkene (1-butene), which is the Hofmann product, although the Zaitsev product (2-butene) is also formed. The ratio between the products depends on reaction condition and steric effects.
Example 5: Electrophilic Addition
Consider the reaction of propene with hydrogen bromide.
CH3CH=CH2 + HBr → ?
This is an electrophilic addition reaction. The hydrogen bromide adds across the double bond following Markovnikov's rule, resulting in the major product being 2-bromopropane (CH3CHBrCH3).
Advanced Considerations: Regioselectivity and Stereoselectivity
Regioselectivity refers to the preference for one regioisomer (product with different position of substituents) over another. Stereoselectivity refers to the preference for one stereoisomer (product with different spatial arrangement of atoms) over another. These concepts are crucial for accurately predicting the major products.
Understanding factors such as steric effects, electronic effects, and transition state geometries allows for more precise predictions.
Conclusion
Predicting the major products of organic reactions is a complex but rewarding skill. By understanding reaction mechanisms, mastering the principles of regioselectivity and stereoselectivity, and considering the various factors influencing product formation, you can confidently approach and solve a wide range of organic chemistry problems. This article has provided a comprehensive framework for this process, illustrating the concepts with several examples. Continued practice and exposure to diverse reaction types are key to further developing this essential skill. Remember to always consider the specific reaction conditions and substrate structure when making predictions. This approach allows for a more nuanced understanding and improved accuracy in determining the predominant products formed. The examples above provide a solid foundation, enabling you to move towards more intricate organic reaction analyses.
Latest Posts
Latest Posts
-
A Preference Decision In Capital Budgeting
Mar 17, 2025
-
If A Company Recognizes Accrued Salary Expense
Mar 17, 2025
-
Utma Accounts Are Opened Under The Tax Id Of The
Mar 17, 2025
-
In Which Situations Can Simplifying Jobs Be Most Beneficial
Mar 17, 2025
-
For The Hr Planning Process How Should Goals Be Determined
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Draw The Major Products Of This Reaction. Ignore Inorganic Byproducts . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
