Draw The Main Lewis Structure Of Nof
Holbox
Mar 26, 2025 · 5 min read
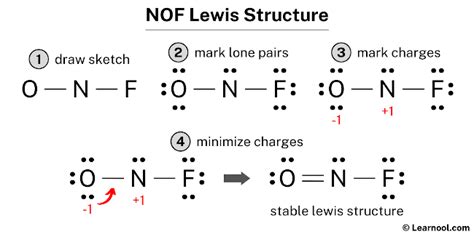
Table of Contents
- Draw The Main Lewis Structure Of Nof
- Table of Contents
- Drawing the Main Lewis Structure of NOF: A Comprehensive Guide
- Understanding Lewis Structures
- Step-by-Step Lewis Structure Construction of NOF
- Why Other Structures Are Less Favourable
- Resonance Structures
- Molecular Geometry and Polarity
- Applications and Significance
- Conclusion
- Latest Posts
- Latest Posts
- Related Post
Drawing the Main Lewis Structure of NOF: A Comprehensive Guide
The seemingly simple molecule NOF, nitrosyl fluoride, presents a fascinating challenge in drawing its Lewis structure. The presence of multiple bonding possibilities and the need to satisfy the octet rule (or expanded octet in some scenarios) lead to several potential structures, but only one represents the most stable and accurate depiction. This article will guide you through the step-by-step process of drawing the main Lewis structure of NOF, explaining the reasoning behind each choice and exploring some common misconceptions.
Understanding Lewis Structures
Before diving into the specifics of NOF, let's briefly review the fundamental principles of Lewis structures. Lewis structures, also known as electron dot diagrams, are visual representations of the valence electrons in a molecule. They help us understand the bonding between atoms and predict the molecule's geometry and properties. Key elements of Lewis structure drawing include:
- Valence Electrons: Determine the number of valence electrons for each atom involved. Nitrogen (N) has 5, Oxygen (O) has 6, and Fluorine (F) has 7.
- Central Atom: Identify the central atom, which is typically the least electronegative atom that can form the most bonds. In NOF, nitrogen is the least electronegative and can form multiple bonds, making it the central atom.
- Octet Rule: Atoms strive to achieve a stable electron configuration with eight valence electrons (except for hydrogen and helium, which aim for two). This is known as the octet rule. However, there are exceptions, particularly with elements in the third period and beyond, which can accommodate more than eight electrons (expanded octet).
- Formal Charge: Calculate the formal charge on each atom to assess the stability of the structure. The formal charge is the difference between the number of valence electrons an atom has in its neutral state and the number of electrons it "owns" in the Lewis structure (assigned as half the bonding electrons plus all non-bonding electrons). Lower formal charges indicate greater stability.
Step-by-Step Lewis Structure Construction of NOF
Here's a step-by-step process to draw the main Lewis structure of NOF:
Step 1: Count the Total Valence Electrons
Nitrogen contributes 5 valence electrons, oxygen contributes 6, and fluorine contributes 7. The total number of valence electrons is 5 + 6 + 7 = 18.
Step 2: Identify the Central Atom
As mentioned earlier, nitrogen is the central atom.
Step 3: Form Single Bonds
Connect the central nitrogen atom to the oxygen and fluorine atoms with single bonds. This uses 4 electrons (2 electrons per bond).
Step 4: Distribute the Remaining Electrons
We have 14 electrons remaining (18 - 4 = 14). Distribute these electrons to satisfy the octet rule for oxygen and fluorine. Oxygen and fluorine are more electronegative than nitrogen. Oxygen and fluorine need 6 more electrons to complete octet each. Therefore, Oxygen and fluorine will each get 6 electrons (3 lone pairs of electrons).
Step 5: Check the Octet Rule
At this stage, oxygen and fluorine have a complete octet. Nitrogen only has 6 electrons which means the Octet rule is not satisfied for nitrogen.
Step 6: Forming Double Bonds to satisfy Octet rule
To complete the octet for nitrogen, we need to form a double bond between nitrogen and one of the other atoms. Since both Oxygen and Fluorine are electronegative, and Oxygen is less electronegative than Fluorine, it is more favorable to form a double bond between Nitrogen and Oxygen. It will minimize formal charge.
Step 7: Final Lewis Structure and Formal Charges
The final Lewis structure will show a double bond between nitrogen and oxygen, and a single bond between nitrogen and fluorine. Oxygen will have two lone pairs, and fluorine will have three lone pairs. Let's calculate the formal charges:
- Nitrogen: 5 (valence electrons) - 4 (non-bonding electrons) - 4 (bonding electrons)/2 = 0
- Oxygen: 6 (valence electrons) - 4 (non-bonding electrons) - 4 (bonding electrons)/2 = 0
- Fluorine: 7 (valence electrons) - 6 (non-bonding electrons) - 2 (bonding electrons)/2 = 0
All atoms have a formal charge of 0, indicating a stable structure.
O
||
N - F
/ \
: :
Why Other Structures Are Less Favourable
Several alternative structures might be considered, but they are less stable due to higher formal charges or incomplete octets. For example, a structure with a double bond between nitrogen and fluorine would result in a positive formal charge on fluorine (a highly electronegative atom), which is energetically unfavorable. Similarly, structures with triple bonds would lead to significant formal charges and are less stable.
Resonance Structures
While the structure presented above is the most stable contributor, it's important to note that resonance structures exist for NOF. Resonance refers to the delocalization of electrons, where the actual electron distribution is a hybrid of multiple contributing structures. Although the double bond is predominantly between nitrogen and oxygen, there's a slight contribution from a resonance structure with a double bond between nitrogen and fluorine. However, the major contributor remains the structure with the N=O double bond due to oxygen's slightly lower electronegativity compared to fluorine and its greater ability to form double bonds without incurring high formal charges.
Molecular Geometry and Polarity
The Lewis structure predicts the molecular geometry of NOF. The central nitrogen atom has two bonding pairs and one lone pair of electrons. According to the VSEPR (Valence Shell Electron Pair Repulsion) theory, this arrangement leads to a bent molecular geometry, similar to the shape of a water molecule. Because of the difference in electronegativity of the atoms, the molecule is also polar.
Applications and Significance
NOF is a relatively unstable compound, used in specific niche applications. Its reactivity is related to its polar nature and its capacity to act as an oxidizing and fluorinating agent. Understanding its Lewis structure is crucial to understanding its chemical behavior and potential use in various chemical reactions.
Conclusion
Drawing the Lewis structure of NOF requires careful consideration of valence electrons, octet rule, and formal charges. While multiple structures could be drawn, only the structure with a nitrogen-oxygen double bond and a nitrogen-fluorine single bond satisfies the octet rule with minimal formal charges, representing the most stable and accurate depiction of the molecule. Understanding this dominant structure is vital to comprehending NOF's properties and reactivity. This in-depth analysis provides a clear and comprehensive understanding of the process, helping readers effectively apply these principles to other molecules and deepening their understanding of chemical bonding. Remember to always prioritize structures with minimal formal charges and complete octets (or the most appropriate expanded octets if necessary) when constructing Lewis structures.
Latest Posts
Latest Posts
-
What Determines Market Price And Equilibrium Output In A Market
Mar 29, 2025
-
Draw The Major E2 Reaction Product Formed When Cis 1 Chloro
Mar 29, 2025
-
At A Price Of 15 There Would Be A
Mar 29, 2025
-
Stockholders In A Publicly Held Corporation Have Limited Liability
Mar 29, 2025
-
How Many Variables Are In The Data Set
Mar 29, 2025
Related Post
Thank you for visiting our website which covers about Draw The Main Lewis Structure Of Nof . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
