Draw A Skeletal Line Structure Of This Molecule
Holbox
Mar 15, 2025 · 5 min read
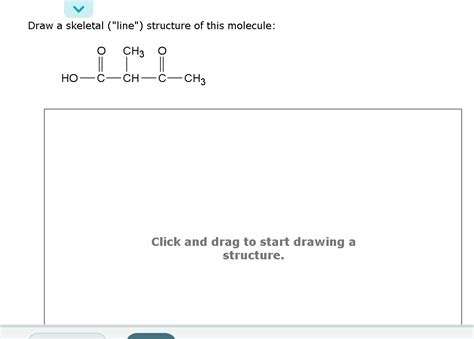
Table of Contents
Drawing Skeletal Line Structures: A Comprehensive Guide
Drawing skeletal line structures, also known as skeletal formulas or condensed structures, is a fundamental skill in organic chemistry. These structures provide a simplified, yet informative, representation of molecules, highlighting the carbon-carbon and carbon-hydrogen bonding framework. Mastering this skill is crucial for understanding organic chemistry concepts, predicting reactivity, and efficiently communicating molecular structures. This comprehensive guide will delve into the intricacies of drawing skeletal line structures, equipping you with the knowledge and practice to confidently represent a vast array of organic molecules.
Understanding the Basics of Skeletal Structures
Before diving into the complexities of drawing skeletal structures, let's solidify our understanding of the fundamental principles:
Carbon Atoms: The Backbone
In skeletal structures, carbon atoms are implied. They are not explicitly drawn as "C," but rather represented by the intersections of lines and the ends of lines. Each intersection or end represents a carbon atom, unless otherwise indicated.
Hydrogen Atoms: Implicitly Present
Hydrogen atoms bonded to carbon atoms are typically omitted. However, it's crucial to understand they are implicitly present. For example, a single line represents a C-C single bond, and each carbon atom carries enough hydrogen atoms to fulfill its four valency.
Heteroatoms: Explicitly Shown
Heteroatoms—atoms other than carbon and hydrogen (like oxygen, nitrogen, sulfur, halogens, etc.)—are explicitly drawn within the skeletal structure using their respective chemical symbols. Their bonds to carbon or other heteroatoms are shown as lines.
Multiple Bonds: Clearly Indicated
Double and triple bonds are indicated by two or three lines connecting atoms, respectively. These explicitly show the presence of pi-bonds.
Step-by-Step Guide to Drawing Skeletal Structures
Let's break down the process of drawing skeletal line structures through a series of examples, increasing in complexity:
Example 1: Simple Alkanes
Consider the simplest alkane, methane (CH₄). In a skeletal structure, this is represented simply as a single point or a dot, implicitly representing the carbon atom with four implicit hydrogen atoms.
Ethane (C₂H₆) is represented by a single line, where each end represents a carbon atom with three implicit hydrogen atoms each.
Propane (C₃H₈) is represented by a chain of three carbons: -C-C-C- Each terminal carbon has three implicit hydrogen atoms, and the central carbon has two.
Example 2: Incorporating Heteroatoms
Let's draw the skeletal structure of ethanol (CH₃CH₂OH). The carbon chain is represented as a two-carbon chain as in ethane. The oxygen atom is explicitly shown, with a bond to the terminal carbon and a separate bond to the hydrogen atom:
CH3-CH2-OH becomes CH3-CH2-O-H becomes CC-O
|
H
Notice how we first draw it with the carbons and oxygens, and then simplified down to the final skeletal form.
Example 3: Branched Alkanes
Drawing branched alkanes requires careful consideration of branching points. Let's consider isobutane [(CH₃)₃CH]:
CH3
|
CH3-C-CH3 becomes C(C)(C)C
|
CH3
The central carbon is shown as the point of intersection where three methyl groups branch.
Example 4: Incorporating Multiple Bonds
Let's draw the skeletal structure of propene (CH₂=CHCH₃):
CH2=CH-CH3 becomes C=CC
The double bond is represented by two lines between the carbons.
Example 5: Cyclic Structures
Cyclic structures are represented as closed loops. Cyclohexane (C₆H₁₂) is a good example:
CH2-CH2
| |
CH2-CH2-CH2-CH2 becomes a hexagon
| |
CH2-CH2
A hexagon represents the six-membered ring.
Example 6: Complex Molecules with Multiple Functional Groups
Now let's consider a more complex molecule, for example, 2-methylbutanoic acid:
CH3 O
| ||
CH3-CH-CH2-C-OH becomes C(C)CCC(=O)O
This structure demonstrates the integration of alkyl groups and carboxylic acid functionalities within a skeletal framework.
Advanced Techniques and Considerations
As you progress in organic chemistry, you'll encounter more complex molecules requiring more advanced techniques:
Stereochemistry: Indicating Chirality
Skeletal structures can be augmented to represent stereochemistry, using wedges and dashes to indicate the spatial arrangement of atoms around chiral centers. A wedge indicates a bond projecting out of the plane of the paper, while a dash represents a bond projecting behind the plane.
Aromatic Rings: Simplified Representation
Aromatic rings, such as benzene, are typically represented by a hexagon with a circle inside to denote the delocalized pi-electron system.
Condensed Structures: A Middle Ground
Condensed structures are a slightly less simplified representation than skeletal structures. They retain some explicit atom representation (especially for heteroatoms and functional groups) but still condense adjacent carbon atoms and their associated hydrogens into a group.
Practice and Resources
Mastering skeletal line structures requires consistent practice. Start with simple molecules and gradually work your way towards more complex ones. Many online resources, textbooks, and practice problems can aid in your learning journey. Focus on understanding the underlying principles and developing a systematic approach to drawing these structures.
The Importance of Accuracy and Clarity
Accurate and clear drawing of skeletal line structures is paramount for effective communication in organic chemistry. Ambiguity can lead to misinterpretations and errors, potentially affecting synthesis, analysis, and other chemical procedures. Pay close attention to detail, ensuring all bonds and atoms are accurately represented, and practice consistently to improve your speed and precision.
Beyond the Basics: Applications in Organic Chemistry
The ability to draw and interpret skeletal structures is fundamental to understanding various organic chemistry concepts:
- Nomenclature: Understanding skeletal structures is crucial for naming organic compounds using IUPAC nomenclature.
- Isomerism: Skeletal structures help in identifying different types of isomers, such as structural isomers, stereoisomers, and conformational isomers.
- Reaction Mechanisms: Visualizing reaction mechanisms through skeletal structures aids in understanding the movement of electrons and the formation and breaking of bonds.
- Spectroscopy: Interpreting spectroscopic data, such as NMR and IR spectra, often requires correlating the spectra to the skeletal structure of the molecule.
- Drug Design and Discovery: In medicinal chemistry, drawing and interpreting skeletal structures is crucial for designing and analyzing drugs.
Conclusion
Drawing skeletal line structures is a cornerstone of organic chemistry, enabling efficient representation and communication of complex molecular structures. By understanding the fundamental principles, mastering the techniques, and consistently practicing, you will develop a crucial skill that will significantly enhance your understanding and proficiency in this vital field of chemistry. Remember to practice regularly, utilizing a variety of examples and progressively increasing the complexity of the molecules you represent. Through consistent effort, you will build confidence and fluency in the powerful language of skeletal structures.
Latest Posts
Latest Posts
-
Cash Flows From Financing Activities Do Not Include
Mar 17, 2025
-
A Positive Return On Investment For Education Happens When
Mar 17, 2025
-
What Is The Value Of I
Mar 17, 2025
-
The Accounts In The Ledger Of Monroe Entertainment Co
Mar 17, 2025
-
A Process Cost Accounting System Is Most Appropriate When
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Draw A Skeletal Line Structure Of This Molecule . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
