Consider The Reaction. Add Curved Arrows For The First Step
Holbox
Mar 19, 2025 · 6 min read
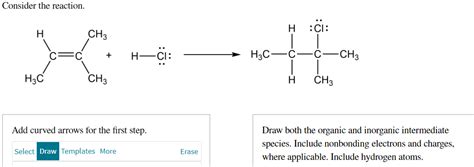
Table of Contents
Consider the Reaction: A Deep Dive into Reaction Mechanisms with Curved Arrows
Understanding reaction mechanisms is fundamental to organic chemistry. It allows us to predict the products of a reaction, understand the factors influencing reaction rates, and design new synthetic routes. A crucial tool in visualizing these mechanisms is the use of curved arrows, which depict the movement of electrons during bond breaking and bond formation. This article will delve into the importance of considering the reaction, specifically focusing on the first step and the crucial role of curved arrows in illustrating electron flow.
The Importance of Considering the Reaction
Before diving into the specifics of curved arrows, let's establish the importance of thoroughly considering the reaction itself. This involves several key aspects:
1. Identifying the Reactants and Products:
The first, and arguably most important, step is accurately identifying all reactants and products involved in the reaction. This may seem obvious, but a thorough understanding of the starting materials and their potential reactivity is crucial for predicting the outcome. Consider the functional groups present, their reactivity, and any potential steric hindrance that might affect the reaction pathway.
Example: A reaction involving a primary alkyl halide and a strong nucleophile will likely proceed via an SN2 mechanism, whereas a tertiary alkyl halide under the same conditions might favor an SN1 mechanism due to steric hindrance.
2. Assessing Reaction Conditions:
Reaction conditions, including solvent, temperature, and the presence of catalysts or other reagents, dramatically influence the reaction pathway. A seemingly simple change in solvent polarity can switch the preferred mechanism from SN1 to SN2, or even lead to unexpected side reactions.
Example: A reaction performed in a protic solvent (like water or methanol) will often favor SN1 reactions due to solvent stabilization of the carbocation intermediate. Conversely, aprotic solvents (like DMSO or DMF) tend to favor SN2 reactions.
3. Understanding Reaction Kinetics and Thermodynamics:
Consider whether the reaction is kinetically or thermodynamically controlled. A kinetically controlled reaction will favor the faster pathway, even if it doesn't lead to the most stable product. A thermodynamically controlled reaction, on the other hand, will favor the most stable product, even if it requires a higher activation energy.
Example: The addition of a strong nucleophile to an electrophilic alkene might initially form a less stable kinetic product, which can then isomerize to a more stable thermodynamic product under suitable conditions.
4. Recognizing Potential Side Reactions:
It's crucial to anticipate potential side reactions that might compete with the desired transformation. This often involves considering the reactivity of various functional groups present in the molecule and understanding the potential for rearrangements or other unexpected transformations.
Example: A reaction involving an epoxide might undergo both ring-opening by a nucleophile and acid-catalyzed ring-opening, leading to a mixture of products.
The Crucial Role of Curved Arrows in Illustrating the First Step
Once the reaction has been carefully considered, curved arrows are used to illustrate the movement of electrons during the reaction mechanism. These arrows are essential for visually representing the bond breaking and bond formation that occur in each step. For the first step specifically, careful consideration is crucial:
1. Identifying the Nucleophile and Electrophile:
The first step often involves the interaction of a nucleophile (electron-rich species) and an electrophile (electron-deficient species). Identifying these species is critical for accurately drawing the curved arrows.
Example: In a nucleophilic substitution reaction, the nucleophile (e.g., OH⁻) will attack the electrophilic carbon atom (e.g., in a alkyl halide).
2. Showing Electron Movement from Nucleophile to Electrophile:
The curved arrow originates from the electron-rich region (often a lone pair of electrons or a pi bond) of the nucleophile and points towards the electron-deficient region (often a partially positive atom or a pi bond) of the electrophile. This indicates the donation of electrons from the nucleophile to the electrophile, initiating the reaction.
Example: In an SN2 reaction, the curved arrow would start from the lone pair of electrons on the oxygen atom of the hydroxide ion and point towards the carbon atom bearing the leaving group in the alkyl halide.
3. Depicting Bond Breaking and Formation:
The curved arrow also shows the breaking of existing bonds and the formation of new bonds. When a bond breaks, the arrow points away from the atom that retains the electrons (heterolytic cleavage) or points to both atoms involved in the bond breaking (homolytic cleavage). When a new bond forms, the arrow points toward the atom that accepts the electron pair.
Example: In an SN2 reaction, one arrow would depict the attack of the nucleophile on the carbon, while another arrow would show the breaking of the carbon-leaving group bond. The leaving group departs with its electron pair.
4. Considering Resonance Structures:
If resonance structures are involved in the first step, the curved arrows should accurately reflect the movement of electrons between these structures. This is particularly important for reactions involving conjugated systems or aromatic compounds.
Examples of First Steps and Curved Arrow Notation:
Let's illustrate these concepts with specific examples.
Example 1: SN2 Reaction
Consider the SN2 reaction between bromomethane (CH₃Br) and hydroxide ion (OH⁻).
First Step: The hydroxide ion acts as a nucleophile, attacking the carbon atom bonded to the bromine atom. Simultaneously, the carbon-bromine bond breaks, with the bromine atom taking the bonding electrons.
Curved Arrows:
.. ..
:O⁻H + H₃C−Br -----> H₃C−O⁻H + Br⁻
.. |
|
|
Curved arrow from the lone pair on oxygen to the carbon.
Another curved arrow from the carbon-bromine bond to the bromine atom.
The first arrow shows the nucleophilic attack, and the second arrow shows the bond breaking and departure of the bromide ion.
Example 2: Electrophilic Aromatic Substitution
Consider the first step of electrophilic aromatic substitution, specifically nitration of benzene.
First Step: The electrophile, nitronium ion (NO₂⁺), attacks the pi electron system of the benzene ring.
Curved Arrows:
+ ..
NO₂⁺ + C₆H₆ -----> Resonance Structures showing positive charge delocalized across the ring.
| / \
| / \
| / \
Curved arrow from the pi electron cloud of the benzene ring to the nitrogen atom of the nitronium ion.
This shows the attack of the electrophile and the delocalization of the positive charge across the benzene ring. This generates a resonance-stabilized carbocation intermediate.
Example 3: Addition to a Carbonyl Group
Consider the first step of a nucleophilic addition reaction to a carbonyl group, such as the reaction of an aldehyde with a Grignard reagent.
First Step: The Grignard reagent acts as a nucleophile, attacking the electrophilic carbonyl carbon.
Curved Arrows:
O O⁻
|| |
R−C−H + R'MgBr -----> R−C−H
| |
| MgBr⁺
|
Curved arrow from the carbon atom of the Grignard reagent to the carbonyl carbon.
Another curved arrow from the carbon-oxygen double bond to the oxygen atom.
This illustrates the nucleophilic attack on the carbonyl carbon and the subsequent formation of a new carbon-carbon bond, and the formation of an alkoxide.
Conclusion:
Mastering the art of considering the reaction and using curved arrows effectively is essential for understanding and predicting the outcome of organic reactions. By carefully analyzing the reactants, reaction conditions, and potential side reactions, and by accurately depicting the movement of electrons with curved arrows, we can gain a deeper understanding of reaction mechanisms and their underlying principles. Remember to practice and develop your skills in drawing reaction mechanisms; it is a crucial skill for success in organic chemistry. The more you practice, the more intuitive the process will become, allowing you to accurately predict reaction pathways and design sophisticated synthetic strategies.
Latest Posts
Latest Posts
-
The Overhead Variance Is The Difference Between
Mar 19, 2025
-
What Is The Formula Mass Of Mg No3 2
Mar 19, 2025
-
Hipaa And Ferpa Prevent A Professionally Mandated Reporter
Mar 19, 2025
-
Which Statement Regarding Free Radicals Is False
Mar 19, 2025
-
The Control Devices Used In Pneumatics Are Called
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about Consider The Reaction. Add Curved Arrows For The First Step . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
