What Is The Formula Mass Of Mg No3 2
Holbox
Mar 19, 2025 · 5 min read
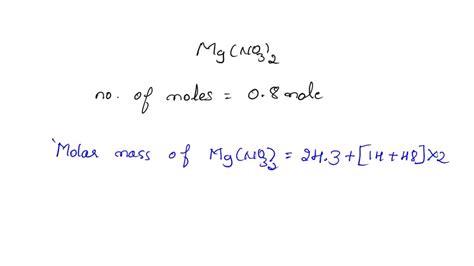
Table of Contents
What is the Formula Mass of Mg(NO₃)₂? A Deep Dive into Calculating and Understanding Formula Mass
Determining the formula mass of a compound is a fundamental concept in chemistry. This article will provide a comprehensive explanation of how to calculate the formula mass of magnesium nitrate, Mg(NO₃)₂, and delve into the underlying principles and applications of this important calculation. We will also explore related concepts such as molar mass and their practical uses in various chemical calculations.
Understanding Formula Mass
Formula mass, also known as formula weight, is the sum of the atomic masses of all the atoms present in a chemical formula. It's expressed in atomic mass units (amu) or daltons (Da). Crucially, it applies to both ionic compounds (like Mg(NO₃)₂) and covalent compounds. Unlike molar mass, which refers to the mass of one mole of a substance, formula mass focuses on the mass of a single formula unit.
Key Difference: Formula Mass vs. Molar Mass: While closely related, formula mass and molar mass differ in their scope. Formula mass describes the mass of a single formula unit, while molar mass describes the mass of one mole (6.022 x 10²³ formula units) of that substance. The numerical value is the same for both, but the units differ (amu vs. g/mol).
Calculating the Formula Mass of Mg(NO₃)₂
To calculate the formula mass of magnesium nitrate, Mg(NO₃)₂, we need to consider the atomic masses of each element present in the compound:
- Magnesium (Mg): Atomic mass ≈ 24.31 amu
- Nitrogen (N): Atomic mass ≈ 14.01 amu
- Oxygen (O): Atomic mass ≈ 16.00 amu
Step-by-Step Calculation:
-
Identify the number of atoms of each element: The formula Mg(NO₃)₂ indicates:
- 1 Magnesium atom (Mg)
- 2 Nitrogen atoms (N) (because of the subscript 2 outside the parenthesis)
- 6 Oxygen atoms (O) (because there are 2 nitrate ions, NO₃, each containing 3 oxygen atoms: 2 x 3 = 6)
-
Multiply the atomic mass of each element by its number of atoms:
- Magnesium: 1 Mg atom x 24.31 amu/Mg atom = 24.31 amu
- Nitrogen: 2 N atoms x 14.01 amu/N atom = 28.02 amu
- Oxygen: 6 O atoms x 16.00 amu/O atom = 96.00 amu
-
Sum the masses of all the atoms:
- Total formula mass = 24.31 amu + 28.02 amu + 96.00 amu = 148.33 amu
Therefore, the formula mass of Mg(NO₃)₂ is approximately 148.33 amu.
Applications of Formula Mass
Understanding and calculating formula mass is crucial in numerous chemical applications:
1. Stoichiometric Calculations:
Formula mass is essential for performing stoichiometric calculations, which involve determining the amounts of reactants and products in a chemical reaction. By using the formula mass, we can convert between mass and moles, allowing us to accurately predict the quantities involved in a reaction.
2. Determining Empirical and Molecular Formulas:
The formula mass helps in determining the empirical and molecular formulas of compounds. If the molar mass of a compound is known experimentally, we can compare it to the calculated formula mass to determine the relationship between the empirical and molecular formulas.
3. Calculating Percentage Composition:
Formula mass plays a vital role in calculating the percentage composition of elements within a compound. Knowing the mass of each element relative to the total formula mass allows us to determine the percentage contribution of each element. This information is crucial in various analytical techniques.
4. Understanding Solution Concentrations:
In solutions, formula mass is critical for preparing solutions of known concentrations. For instance, to prepare a solution of a specific molarity, we need to know the formula mass to calculate the required mass of solute.
5. Analyzing Chemical Reactions:
Formula mass helps in analyzing the changes in mass during chemical reactions. By comparing the formula masses of reactants and products, we can understand the mass relationships involved, and confirm that the Law of Conservation of Mass is upheld.
Significance of Precision in Atomic Masses
The accuracy of the calculated formula mass depends heavily on the precision of the atomic masses used. Atomic masses are not whole numbers because they represent the weighted average of the isotopes of each element. Using more precise atomic masses from a periodic table will yield a more precise formula mass. For most general chemistry calculations, the atomic masses rounded to two decimal places are sufficient. However, for high-precision work, more significant figures are required.
Further Exploration: Molar Mass and its Relationship to Formula Mass
As mentioned earlier, molar mass is the mass of one mole (6.022 x 10²³) of a substance. Its numerical value is identical to the formula mass, but its units are grams per mole (g/mol). The relationship between formula mass and molar mass is fundamental in chemistry, facilitating the conversion between mass and moles in various calculations.
Real-World Applications of Magnesium Nitrate
Magnesium nitrate, Mg(NO₃)₂, finds applications in various fields:
- Agriculture: It's used as a fertilizer due to its magnesium and nitrogen content, essential nutrients for plant growth.
- Pyrotechnics: It acts as an oxidizing agent in some fireworks, contributing to their bright light and color.
- Industrial Processes: It finds use in various industrial processes, such as in the production of certain chemicals and materials.
Conclusion:
Calculating the formula mass of Mg(NO₃)₂ (and other compounds) is a fundamental skill in chemistry. Understanding this concept lays the groundwork for more complex calculations in stoichiometry, solution chemistry, and other areas of chemical study. The precise calculation of formula mass, using accurate atomic masses, is crucial for achieving accurate results in various scientific and industrial applications. The applications of Mg(NO₃)₂ further highlight the practical significance of understanding this essential chemical concept. Remember to always use a reliable periodic table for the most accurate atomic masses to ensure the precision of your calculations.
Latest Posts
Latest Posts
-
Electric Potential Very Connfusing Multiple Choice Question
Mar 19, 2025
-
Incident Objectives That Drive Incident Operations Are Established By The
Mar 19, 2025
-
Label The Structures Of The Pelvis
Mar 19, 2025
-
Environmental Scanning Is Necessary For An Organization To
Mar 19, 2025
-
Which Of These Are Examples Of Business Analytics
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about What Is The Formula Mass Of Mg No3 2 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
