Consider The Face-centered Cubic Unit Cell Shown In This Image
Holbox
Mar 19, 2025 · 7 min read
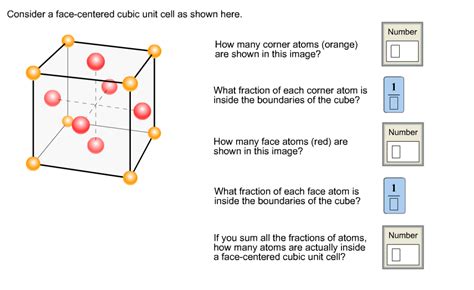
Table of Contents
Decoding the Face-Centered Cubic (FCC) Unit Cell: A Deep Dive
The face-centered cubic (FCC) unit cell, a fundamental building block in materials science and crystallography, represents a crucial arrangement of atoms within a crystalline structure. Understanding its properties, characteristics, and implications is essential for comprehending the behavior of numerous materials, from metals to ceramics. This article will comprehensively explore the FCC unit cell, delving into its structure, coordination number, atomic packing factor, properties, examples, and applications.
Understanding the Structure of the FCC Unit Cell
The FCC unit cell is characterized by its unique atomic arrangement. Imagine a cube; in an FCC structure, atoms reside at each of the eight corners of the cube, and importantly, at the center of each of the six faces. This arrangement differs significantly from the body-centered cubic (BCC) structure, where an additional atom occupies the very center of the cube. The atoms at the corners are shared between eight adjacent unit cells, contributing only 1/8th of their volume to each cell. The atoms on the faces are shared between two adjacent unit cells, each contributing 1/2 of their volume.
Key Features of the FCC Structure
-
Atoms per Unit Cell: A careful consideration reveals that a single FCC unit cell contains a total of four atoms. (8 corner atoms * 1/8 atom/corner) + (6 face atoms * 1/2 atom/face) = 4 atoms.
-
Coordination Number: The coordination number refers to the number of nearest-neighbor atoms surrounding a central atom. In an FCC structure, the coordination number is 12. Each atom is in contact with twelve neighboring atoms. This high coordination number contributes to the relatively high density of FCC materials.
-
Atomic Packing Factor (APF): The APF represents the fraction of volume in a unit cell that is actually occupied by atoms. For an FCC structure, the APF is remarkably high at approximately 74%. This means that 74% of the unit cell's volume is filled with atoms, leaving only 26% as void space. This efficient packing arrangement contributes to the strength and density of many FCC metals. The calculation involves determining the volume occupied by atoms and dividing it by the total volume of the unit cell.
-
Nearest Neighbor Distance: The distance between the centers of two nearest-neighbor atoms is critical in determining material properties. In an FCC structure, this distance is related to the lattice parameter (the edge length of the cube, often denoted as 'a').
Deriving the Relationship between Lattice Parameter and Atomic Radius
The relationship between the lattice parameter (a) and the atomic radius (r) is fundamental to understanding the geometry of the FCC structure. Consider the atoms on a face diagonal. The face diagonal is composed of four atomic radii (2r + 2r). Using Pythagorean theorem on a face, the relationship can be established as:
4r² = 2a²
Solving for 'a', we get:
a = 2√2r
This equation highlights the direct proportionality between the lattice parameter and the atomic radius in an FCC structure. Knowing one value allows for the easy calculation of the other. This relationship is crucial for various applications, including determining the density of a material based on its atomic weight and radius.
Calculating Density of FCC Materials
Density, a critical property of materials, is directly linked to the atomic arrangement and other material properties. For an FCC structure, the density (ρ) can be calculated using the following formula:
ρ = (nA)/(V<sub>c</sub>N<sub>A</sub>)
Where:
- n = number of atoms per unit cell (4 for FCC)
- A = atomic weight
- V<sub>c</sub> = volume of the unit cell (a³)
- N<sub>A</sub> = Avogadro's number (6.022 x 10²³ atoms/mol)
By substituting the expression for 'a' derived earlier (a = 2√2r), the density equation can be expressed solely in terms of atomic radius and atomic weight, further solidifying the relationship between atomic-level parameters and macroscopic properties.
Properties and Characteristics of FCC Metals
FCC metals exhibit several distinct properties stemming from their unique atomic arrangement:
-
Ductility and Malleability: The close-packed arrangement and high coordination number in FCC structures allow for relatively easy deformation under stress. This results in high ductility (ability to be drawn into wires) and malleability (ability to be shaped into sheets). This contrasts with BCC structures, which are generally less ductile.
-
High Density: The efficient atomic packing (74% APF) results in high density compared to other crystal structures.
-
Slip Systems: FCC structures possess multiple slip systems, contributing to their ductility. Slip systems are planes along which atoms can easily slide past each other under stress. The existence of many slip systems in FCC materials allows them to deform plastically under stress without fracturing.
-
Stacking Fault Energy: FCC metals typically have relatively low stacking fault energy. Stacking faults are deviations from the ideal FCC stacking sequence. This characteristic impacts their mechanical properties and is crucial in considering their behavior during plastic deformation.
-
Solid Solution Strengthening: The ability to form substitutional solid solutions with other elements often enhances the strength and hardness of FCC metals without significant loss of ductility. This is a key aspect in alloy design and development.
Examples of Materials with FCC Crystal Structure
Many common metals and alloys exhibit the FCC crystal structure. Notable examples include:
-
Aluminum (Al): A lightweight metal with excellent corrosion resistance, widely used in aerospace and automotive industries.
-
Copper (Cu): An excellent conductor of electricity, used extensively in electrical wiring and various applications.
-
Gold (Au): A precious metal prized for its beauty and inertness.
-
Nickel (Ni): A strong, corrosion-resistant metal often used in alloys for high-temperature applications.
-
Lead (Pb): Traditionally used in plumbing and batteries, though its applications are increasingly limited due to environmental concerns.
-
Silver (Ag): Another excellent electrical conductor, frequently employed in electronics and jewelry.
-
Austenitic Stainless Steels: A class of stainless steels that owe their properties to their FCC structure. They are known for their corrosion resistance and ductility.
Applications of FCC Materials
The diverse properties of FCC materials translate to a wide range of applications:
-
Aerospace: Aluminum alloys, owing to their lightweight and strong nature, are extensively used in aircraft and spacecraft construction.
-
Automotive: Steel alloys, including austenitic stainless steels, play significant roles in automotive manufacturing, offering corrosion resistance and strength.
-
Electronics: Copper and silver, with their high electrical conductivity, are essential in electronics and electrical systems.
-
Medical Implants: Certain biocompatible alloys with FCC structure are used in medical implants due to their corrosion resistance and biocompatibility.
-
Packaging: Aluminum's corrosion resistance makes it a popular choice for food and beverage packaging.
-
Jewelry: Gold and silver's aesthetic appeal and inertness make them staples in jewelry manufacturing.
Advanced Considerations and Future Research
While the basic understanding of the FCC unit cell is relatively straightforward, ongoing research continues to explore more nuanced aspects:
-
Defect Structures: Defects within the FCC lattice, such as vacancies, dislocations, and stacking faults, significantly influence material properties. Research continues to elucidate the role of these defects in enhancing or diminishing material performance.
-
Alloying Effects: Understanding how different alloying elements modify the properties of FCC metals is an active field of investigation. Tailoring alloy compositions to achieve specific properties is crucial in materials design.
-
Nanostructured FCC Materials: The synthesis and characterization of nanostructured FCC materials is an emerging area, exploring the unique properties that arise at the nanoscale.
-
Computational Modeling: Computational techniques, such as density functional theory (DFT), are being increasingly employed to predict and understand the properties of FCC materials, complementing experimental approaches.
-
Phase Transformations: Understanding phase transformations involving FCC structures is vital, as they often dictate material behavior at different temperatures and pressures.
In conclusion, the face-centered cubic unit cell represents a critical and versatile crystal structure with far-reaching implications in materials science and engineering. Its unique atomic arrangement, high packing efficiency, and associated properties contribute to the wide range of applications of FCC materials, making it a key concept for understanding the behavior and performance of numerous materials in diverse technological fields. Ongoing research continues to unveil further complexities and functionalities associated with FCC structures, promising exciting advancements in materials design and applications.
Latest Posts
Latest Posts
-
In Which Situation Is A Combining Vowel Never Used
Mar 19, 2025
-
When The Wash Sale Rules Apply The Realized Loss Is
Mar 19, 2025
-
Fixed Costs Expressed On A Per Unit Basis
Mar 19, 2025
-
A Companys Strategic Plan Consists Of
Mar 19, 2025
-
Once The Estimated Depreciation Expense For An Asset Is Calculated
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about Consider The Face-centered Cubic Unit Cell Shown In This Image . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
