Complete The Electron Pushing Mechanism For The Given Decarboxylation Reaction
Holbox
Mar 22, 2025 · 5 min read
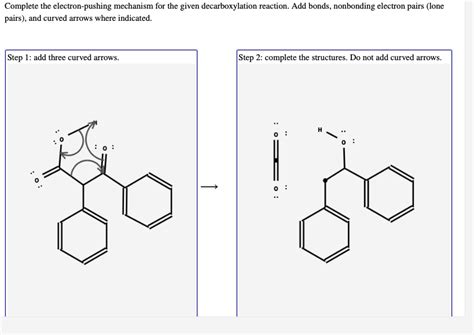
Table of Contents
- Complete The Electron Pushing Mechanism For The Given Decarboxylation Reaction
- Table of Contents
- Complete Electron Pushing Mechanism for Decarboxylation Reactions
- Understanding the Basics of Decarboxylation
- Factors Influencing Decarboxylation
- Electron Pushing Mechanisms for Different Decarboxylation Types
- 1. Decarboxylation of β-Keto Acids
- 2. Decarboxylation of Malonic Acid Derivatives
- 3. Decarboxylation of Aromatic Carboxylic Acids
- 4. Decarboxylative Cross-Coupling Reactions
- 5. Enzymatic Decarboxylation
- Importance of Understanding the Mechanism
- Conclusion
- Latest Posts
- Latest Posts
- Related Post
Complete Electron Pushing Mechanism for Decarboxylation Reactions
Decarboxylation, the process of removing a carboxyl group (-COOH) from a molecule to release carbon dioxide (CO2), is a fundamental reaction in organic chemistry with widespread applications in various fields, from biosynthesis to industrial chemical synthesis. Understanding the electron-pushing mechanism underlying this reaction is crucial for predicting reaction outcomes and designing efficient synthetic strategies. This article provides a comprehensive exploration of the electron pushing mechanisms involved in decarboxylation reactions, covering different reaction types and influencing factors.
Understanding the Basics of Decarboxylation
Before delving into the mechanisms, let's briefly revisit the concept of decarboxylation. The general reaction involves the removal of a carboxyl group (-COOH) from a carboxylic acid or its derivative, resulting in the formation of carbon dioxide (CO2) and a new carbon-containing compound. The ease with which a carboxyl group undergoes decarboxylation depends heavily on the structure of the molecule and the reaction conditions.
Factors Influencing Decarboxylation
Several factors significantly influence the rate and feasibility of decarboxylation reactions:
-
Stability of the resulting carbanion or carbocation: Decarboxylation often proceeds through the formation of a carbanion (negative charge on carbon) or a carbocation (positive charge on carbon). The stability of this intermediate directly impacts the reaction rate. Resonance stabilization, inductive effects, and hyperconjugation all contribute to carbanion/carbocation stability. More stable intermediates lead to faster decarboxylation.
-
Presence of electron-withdrawing groups: Electron-withdrawing groups (EWGs) on the carbon atom adjacent to the carboxyl group can stabilize the developing negative charge during decarboxylation, facilitating the reaction. Examples include nitro (-NO2), cyano (-CN), and carbonyl groups (C=O).
-
Reaction temperature and pH: Decarboxylation reactions are often temperature-dependent. Higher temperatures generally accelerate the reaction. The pH of the reaction medium also plays a critical role, especially for reactions involving carboxylic acids.
-
Presence of a leaving group: In some decarboxylation reactions, a good leaving group (LG) beta to the carboxyl group can facilitate the reaction. The leaving group assists in the formation of a double bond or a stable carbocation.
Electron Pushing Mechanisms for Different Decarboxylation Types
Decarboxylation can proceed through various mechanisms, depending on the substrate and reaction conditions. Let's explore some of the most common mechanisms, illustrating them with electron-pushing arrows.
1. Decarboxylation of β-Keto Acids
This is perhaps the most well-known and frequently encountered type of decarboxylation. β-keto acids, possessing a carbonyl group (C=O) beta to the carboxyl group, readily undergo decarboxylation, often under relatively mild conditions. The mechanism involves the following steps:
-
Protonation of the carbonyl oxygen: A proton from a suitable acid (e.g., H3O+) protonates the carbonyl oxygen, making it a better leaving group.
-
Resonance stabilization: The protonated carbonyl oxygen can then donate its electrons to the carbonyl carbon, forming a resonance-stabilized enol intermediate. This intermediate has a negative charge delocalized between the oxygen and the carbon alpha to the carboxyl group.
-
Decarboxylation: The carboxyl group departs as CO2, leaving behind a resonance-stabilized enolate anion.
-
Protonation: The enolate anion accepts a proton to form a ketone.
Electron Pushing Diagram (β-keto acid decarboxylation):
O O
|| |
H3O+ + CH3-C-CH2-C-OH -----> CH3-C-CH2-C-OH2+
| |
O O
|
| (Electron pair shifts)
v
O O-
|| ||
CH3-C-CH=C-OH <-----> CH3-C-CH=C-OH
| |
O O
| (CO2 leaves)
v
CH3-C-CH2- -----> CH3-C-CH3
| |
O- O
^
| (Protonation)
H+
2. Decarboxylation of Malonic Acid Derivatives
Malonic acid derivatives, containing a dicarboxylic acid moiety, can also undergo decarboxylation under specific conditions. The mechanism often involves the formation of a stabilized carbanion.
Electron Pushing Diagram (Malonic acid derivative decarboxylation - simplified):
HOOC-CH2-COOH --heat--> CH3COOH + CO2
(This is a simplified representation. The full mechanism would involve a detailed description of the carbanion formation and stabilization.)
3. Decarboxylation of Aromatic Carboxylic Acids
Aromatic carboxylic acids, under specific conditions (often high temperature and the presence of strong acids or bases), can undergo decarboxylation. The mechanism often involves the formation of an aryl cation intermediate, which is then stabilized by the aromatic ring.
4. Decarboxylative Cross-Coupling Reactions
These reactions combine decarboxylation with a cross-coupling reaction, resulting in the formation of a new carbon-carbon bond. A palladium catalyst is commonly used to facilitate this process. The mechanism is more complex and involves several steps, including oxidative addition, transmetalation, and reductive elimination.
5. Enzymatic Decarboxylation
In biological systems, enzymatic decarboxylation plays a vital role in metabolic pathways. Enzymes specifically catalyze the decarboxylation of various substrates, often through a mechanism involving the formation of a stable intermediate bound to the enzyme's active site. The specific mechanism varies depending on the enzyme and substrate.
Importance of Understanding the Mechanism
Understanding the electron pushing mechanism of decarboxylation is crucial for several reasons:
-
Predicting reaction outcomes: By understanding the mechanism, one can predict whether a given molecule will undergo decarboxylation and the nature of the products formed.
-
Optimizing reaction conditions: The mechanism guides the optimization of reaction conditions, such as temperature, pH, and the presence of catalysts, to maximize the yield and selectivity of the reaction.
-
Designing new synthetic strategies: A deep understanding of decarboxylation mechanisms enables the design of new synthetic routes for preparing valuable molecules, utilizing decarboxylation as a key step.
-
Understanding biological processes: Understanding the mechanisms of enzymatic decarboxylation helps unravel the complexities of metabolic pathways and their regulation.
Conclusion
Decarboxylation is a versatile and important reaction in organic chemistry with diverse applications. The reaction mechanisms, while varied depending on the substrate and conditions, generally involve the formation of a stabilized carbanion or carbocation intermediate. A thorough understanding of the electron-pushing mechanisms involved is essential for predicting reaction outcomes, optimizing reaction conditions, and designing novel synthetic strategies. Further research continues to expand our knowledge of decarboxylation mechanisms and their applications in various fields, promising exciting advancements in organic chemistry and related disciplines. This comprehensive exploration provides a strong foundation for those seeking to master this fundamental organic reaction.
Latest Posts
Latest Posts
-
What Is The Expected Major Product For The Following Reaction
Mar 24, 2025
-
You Charge An Initially Uncharged Capacitor Through Resistor
Mar 24, 2025
-
How Can Firm Specific Risk Be Defined
Mar 24, 2025
-
Filtrate First Passes From The Glomerular Capsule To The
Mar 24, 2025
-
Mathematics For The Trades Robert A Carman Table Of Contents
Mar 24, 2025
Related Post
Thank you for visiting our website which covers about Complete The Electron Pushing Mechanism For The Given Decarboxylation Reaction . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
