Cis 1 Tert Butyl 4 Methylcyclohexane
Holbox
Mar 26, 2025 · 5 min read
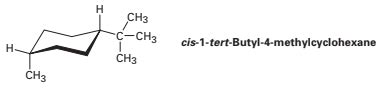
Table of Contents
- Cis 1 Tert Butyl 4 Methylcyclohexane
- Table of Contents
- cis-1-tert-Butyl-4-methylcyclohexane: A Deep Dive into Conformation and Stereochemistry
- Understanding the Structure: cis-1-tert-Butyl-4-methylcyclohexane
- Conformational Analysis: Chair Conformations and Steric Effects
- The Dominant Conformation: Equatorial tert-Butyl
- Methyl Group Position: Axial or Equatorial?
- Energy Considerations: Calculating the Relative Stability
- Comparing cis and trans Isomers: A Critical Distinction
- NMR Spectroscopy: Experimental Evidence for Conformation
- Applications and Significance
- Conclusion: A Powerful Teaching Tool
- Latest Posts
- Latest Posts
- Related Post
cis-1-tert-Butyl-4-methylcyclohexane: A Deep Dive into Conformation and Stereochemistry
Introduction:
cis-1-tert-Butyl-4-methylcyclohexane is a fascinating organic molecule that provides a rich case study for understanding conformational analysis and stereochemistry. Its seemingly simple structure belies a complex interplay of steric interactions and conformational preferences, making it an ideal subject for exploring fundamental concepts in organic chemistry. This article will delve into the detailed structural analysis, conformational equilibria, and the influence of steric effects on its properties. We will also explore its synthesis, although specific synthetic routes will not be detailed here due to the complexity and variability of synthesis procedures. The focus will remain on understanding the molecule's behavior and properties.
Understanding the Structure: cis-1-tert-Butyl-4-methylcyclohexane
The name itself reveals key structural features. Let's break it down:
- cyclohexane: This indicates a six-membered carbon ring, forming a saturated hydrocarbon.
- 1-tert-butyl: A tert-butyl group (–C(CH₃)₃) is attached to carbon number 1 in the cyclohexane ring.
- 4-methyl: A methyl group (–CH₃) is attached to carbon number 4.
- cis: This crucial prefix specifies the relative stereochemistry of the tert-butyl and methyl groups. They both lie on the same side of the cyclohexane ring plane.
This cis configuration has profound implications for the molecule's conformation, as we will explore further.
Conformational Analysis: Chair Conformations and Steric Effects
Cyclohexane predominantly exists in two chair conformations that interconvert rapidly at room temperature. However, the presence of bulky substituents like tert-butyl and methyl significantly impacts this equilibrium. The tert-butyl group, being considerably larger than the methyl group, exerts a dominant influence.
The Dominant Conformation: Equatorial tert-Butyl
The tert-butyl group strongly prefers the equatorial position. Placing it in the axial position leads to severe 1,3-diaxial interactions with the axial hydrogens on carbons 3 and 5. These interactions are highly unfavorable due to significant steric hindrance. Consequently, the conformation with the tert-butyl group equatorial is overwhelmingly favored.
Methyl Group Position: Axial or Equatorial?
The methyl group's position is more nuanced. While it also prefers the equatorial position to minimize steric interactions, the already established equatorial tert-butyl group influences its preference. The cis relationship necessitates that both substituents are on the same side of the ring.
Therefore, while the ideal scenario would be both groups equatorial (which is impossible in a cis isomer), the dominant conformation will have the tert-butyl group equatorial and the methyl group axial. This arrangement minimizes overall steric strain despite the axial methyl group.
Energy Considerations: Calculating the Relative Stability
Precise energy calculations require sophisticated computational chemistry methods (e.g., Density Functional Theory – DFT), which are beyond the scope of this discussion. However, we can qualitatively assess the relative stability of different conformations based on the steric interactions present.
The conformation with the equatorial tert-butyl and axial methyl is significantly more stable than any other possible conformation for the cis isomer. The large steric penalty associated with an axial tert-butyl group completely outweighs the smaller penalty associated with an axial methyl group.
Comparing cis and trans Isomers: A Critical Distinction
It's crucial to compare the cis isomer with its trans counterpart, trans-1-tert-butyl-4-methylcyclohexane. In the trans isomer, the tert-butyl and methyl groups are on opposite sides of the ring. This allows for a conformation where both groups are equatorial, resulting in minimal steric strain and exceptional stability. The trans isomer will overwhelmingly favor this conformation, making it significantly more stable than the cis isomer. This difference in stability highlights the importance of stereochemistry in determining molecular properties.
NMR Spectroscopy: Experimental Evidence for Conformation
Nuclear Magnetic Resonance (NMR) spectroscopy provides powerful experimental evidence to support the conformational analysis. The chemical shifts and coupling constants observed in the ¹H NMR spectrum are consistent with the dominant conformation having an equatorial tert-butyl and an axial methyl group. Detailed analysis of the spectrum can help confirm the preferred conformation and further quantify the energy difference between different conformations.
Applications and Significance
While cis-1-tert-butyl-4-methylcyclohexane may not have widespread industrial applications in the same way as some other organic compounds, its importance lies in its pedagogical value. It serves as an excellent example to illustrate several key concepts in organic chemistry, including:
- Conformational analysis: Understanding the influence of steric effects on molecular conformation.
- Stereochemistry: Differentiating between cis and trans isomers and their distinct properties.
- Energy minimization: Predicting the most stable conformations based on steric interactions.
- NMR spectroscopy: Interpreting spectral data to gain structural information.
These fundamental concepts are crucial for understanding the behavior of a vast range of organic molecules and are essential for advanced studies in organic chemistry, medicinal chemistry, and materials science.
Conclusion: A Powerful Teaching Tool
cis-1-tert-Butyl-4-methylcyclohexane, although a relatively simple molecule, offers a rich landscape for exploring intricate conformational and stereochemical principles. Its study reinforces the importance of considering steric interactions and their profound influence on molecular stability and properties. The molecule's pedagogical value in illustrating these key concepts solidifies its significance in organic chemistry education and research. Its conformational preferences and the comparison with its trans isomer provide a powerful tool for students to grasp complex ideas in a concrete and easily visualizable context. Further investigations using computational chemistry could provide more detailed insights into the energetics and dynamics of the conformational equilibria.
Latest Posts
Latest Posts
-
What Determines Market Price And Equilibrium Output In A Market
Mar 29, 2025
-
Draw The Major E2 Reaction Product Formed When Cis 1 Chloro
Mar 29, 2025
-
At A Price Of 15 There Would Be A
Mar 29, 2025
-
Stockholders In A Publicly Held Corporation Have Limited Liability
Mar 29, 2025
-
How Many Variables Are In The Data Set
Mar 29, 2025
Related Post
Thank you for visiting our website which covers about Cis 1 Tert Butyl 4 Methylcyclohexane . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
