All Amino Acids Have Two Ionizable Functional Groups
Holbox
Mar 19, 2025 · 5 min read
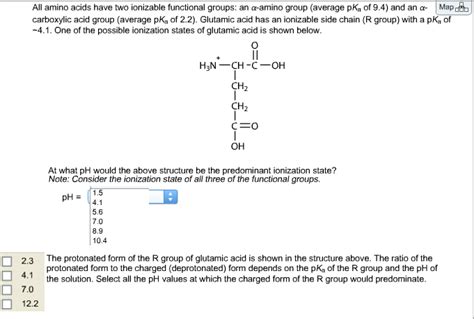
Table of Contents
All Amino Acids Have Two Ionizable Functional Groups: A Deep Dive into Acid-Base Chemistry and Biological Significance
Amino acids, the fundamental building blocks of proteins, possess a remarkable chemical property: they all contain at least two ionizable functional groups. This seemingly simple fact underpins a wealth of their crucial biological functions, from protein folding and enzyme catalysis to cellular signaling and immune responses. Understanding the ionization behavior of these groups is key to comprehending the intricate chemistry of life. This article will delve into the details of these ionizable groups, their pKa values, the concept of isoelectric points (pI), and the broader biological implications of amino acid ionization.
The Two (or More!) Ionizable Groups: A Closer Look
The defining characteristic of amino acids is their possession of both an amino group (-NH₂) and a carboxyl group (-COOH). These two groups are inherently ionizable, meaning they can readily gain or lose a proton (H⁺) depending on the pH of their environment.
The Carboxyl Group (-COOH): An Acidic Affair
The carboxyl group, a carboxylic acid functional group, readily loses a proton in an aqueous solution, behaving as an acid. This deprotonation results in the formation of a carboxylate anion (-COO⁻). The tendency of a carboxyl group to donate a proton is quantified by its pKa value, which typically falls within the range of 2-3 for most amino acids. A lower pKa indicates a stronger acid, meaning it more readily loses its proton.
Understanding pKa: The pKa is the pH at which half of the molecules of a given acid are in their protonated form and half are in their deprotonated form. It’s a crucial parameter in predicting the ionization state of a molecule at a specific pH.
The Amino Group (-NH₂): A Basic Standpoint
In contrast to the carboxyl group, the amino group acts as a base, readily accepting a proton in aqueous solution. This protonation forms a positively charged ammonium ion (-NH₃⁺). The pKa value for the amino group typically ranges from 9-10 for most amino acids. A higher pKa indicates a weaker acid (and thus a stronger base), meaning it less readily donates its proton (or more readily accepts one).
Beyond the Basics: Side Chain Ionization
While the amino and carboxyl groups are common to all amino acids, the story doesn't end there. Many amino acids possess additional ionizable groups within their side chains (R-groups). These side chains exhibit a vast diversity in their chemical properties, leading to a wide range of pKa values.
For instance:
- Aspartic acid (Asp) and Glutamic acid (Glu): Possess additional carboxyl groups in their side chains, exhibiting lower pKa values (around 4) compared to the alpha-carboxyl group.
- Lysine (Lys), Arginine (Arg), and Histidine (His): Contain additional amino groups in their side chains, with varying pKa values reflecting their different basic strengths. Histidine's side chain imidazole group has a pKa near neutral pH (around 6), making it particularly important in enzyme active sites.
- Tyrosine (Tyr) and Cysteine (Cys): Have phenolic hydroxyl (-OH) and thiol (-SH) groups respectively, exhibiting pKa values around 10 and 8.
The Isoelectric Point (pI): The Point of Neutrality
The isoelectric point (pI) represents the pH at which an amino acid carries a net zero charge. At this pH, the positive and negative charges on the molecule exactly balance each other out. The pI value is crucial in various biochemical techniques, such as isoelectric focusing, where amino acids and proteins are separated based on their pI values.
Calculating the pI involves determining the average of the pKa values that flank the zwitterionic form. For amino acids with only two ionizable groups (the α-amino and α-carboxyl), the pI is simply the average of the pKa of the carboxyl group and the pKa of the amino group. However, for amino acids with ionizable side chains, the calculation becomes slightly more complex, requiring consideration of all relevant pKa values.
The Biological Significance of Ionizable Groups
The ionizable nature of amino acids is not merely a chemical curiosity; it's central to their biological roles. Their ionization states significantly influence:
1. Protein Structure and Folding
The electrostatic interactions between ionizable groups play a vital role in protein folding and stability. Attractive forces between oppositely charged groups (e.g., between a positively charged lysine side chain and a negatively charged aspartate side chain) contribute to the formation of specific protein conformations. Conversely, repulsive forces between similarly charged groups can influence protein folding pathways and stability.
2. Enzyme Catalysis
Many enzymes utilize the ionizable groups of amino acids in their active sites to facilitate catalytic reactions. The precise positioning and ionization state of these groups are crucial for substrate binding, orientation, and the chemical transformations that occur during catalysis. Changes in pH can drastically alter the catalytic activity of an enzyme by altering the ionization states of its active site residues.
3. Protein-Protein Interactions
Ionizable groups also play a crucial role in mediating protein-protein interactions. Electrostatic interactions between the charged groups of interacting proteins contribute to the specificity and affinity of these interactions. For instance, the binding of antibodies to antigens often relies on electrostatic interactions between charged amino acid residues on the antibody and antigen surfaces.
4. Cellular Signaling
Many cellular signaling pathways involve proteins that undergo changes in their ionization state upon binding to ligands or other signaling molecules. These changes can trigger conformational shifts, leading to altered protein activity or the initiation of downstream signaling cascades. For example, changes in the phosphorylation state of serine, threonine, or tyrosine residues (which introduces a negative charge) can alter protein function and signaling.
5. pH Homeostasis and Buffering
Amino acids, particularly those with ionizable side chains, contribute to the buffering capacity of biological systems. Their ability to either donate or accept protons helps maintain a relatively stable pH within cells and tissues, despite fluctuations in the concentration of acids and bases.
Conclusion: A Foundation of Life's Chemistry
The presence of at least two ionizable functional groups is a cornerstone of amino acid chemistry and biology. Their ability to gain or lose protons allows them to participate in a vast array of crucial biological processes, from protein folding and enzyme catalysis to cellular signaling and pH homeostasis. Understanding the intricacies of amino acid ionization is fundamental to grasping the complexity and elegance of life's chemistry. Further research into the specific pKa values and ionization behaviors of various amino acids in diverse environments will undoubtedly unveil even more profound insights into the intricate mechanisms of biological systems.
Latest Posts
Latest Posts
-
Absolute Advantage Is Found By Comparing Different Producers
Mar 19, 2025
-
At A Team Meeting The Restaurant Manager
Mar 19, 2025
-
All Of The Following Are Heart Valves Except
Mar 19, 2025
-
Draw The Correct Organic Product Of The Oxidation Reaction Shown
Mar 19, 2025
-
Budget Compare Actual Results To Budgeted Results
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about All Amino Acids Have Two Ionizable Functional Groups . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
