Draw The Correct Organic Product Of The Oxidation Reaction Shown
Holbox
Mar 19, 2025 · 6 min read
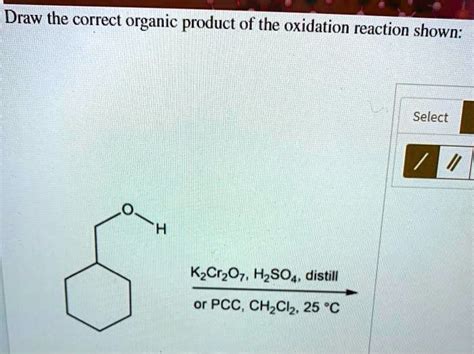
Table of Contents
Drawing the Correct Organic Product of Oxidation Reactions: A Comprehensive Guide
Oxidation reactions are fundamental in organic chemistry, transforming functional groups and altering the properties of molecules. Accurately predicting the product of an oxidation reaction requires a solid understanding of the oxidizing agent used, the substrate's structure, and the reaction conditions. This comprehensive guide will delve into the intricacies of oxidation reactions, equipping you with the knowledge to confidently draw the correct organic product.
Understanding Oxidation in Organic Chemistry
Before diving into specific examples, let's establish a clear understanding of oxidation in the context of organic chemistry. Oxidation is generally defined as an increase in the oxidation state of a carbon atom. This often involves gaining bonds to more electronegative atoms (like oxygen) or losing bonds to less electronegative atoms (like hydrogen). Conversely, reduction involves the opposite—a decrease in oxidation state.
Several oxidizing agents are commonly employed in organic chemistry, each exhibiting varying strengths and selectivities. The choice of oxidizing agent significantly impacts the final product. Let's explore some common ones:
1. Potassium Permanganate (KMnO₄): A strong oxidizing agent capable of oxidizing a wide range of functional groups. It's often used in basic conditions and can cleave carbon-carbon double bonds.
2. Chromic Acid (H₂CrO₄): Another potent oxidizing agent, typically employed in acidic conditions. It's frequently used for the oxidation of alcohols to ketones or carboxylic acids.
3. Jones Reagent (CrO₃ in H₂SO₄): A variation of chromic acid, known for its effectiveness in oxidizing primary alcohols to carboxylic acids.
4. PCC (Pyridinium Chlorochromate): A milder oxidizing agent compared to chromic acid, often used for the selective oxidation of primary alcohols to aldehydes.
5. Swern Oxidation: This method utilizes DMSO and oxalyl chloride to oxidize alcohols to aldehydes or ketones. It's known for its mild conditions and its ability to minimize over-oxidation.
6. Oppenauer Oxidation: A selective oxidation of secondary alcohols to ketones using aluminum isopropoxide.
Predicting Oxidation Products: A Step-by-Step Approach
Predicting the product of an oxidation reaction requires a systematic approach. Here's a breakdown of the steps involved:
1. Identify the Substrate and Oxidizing Agent: Carefully examine the structure of the starting material (substrate) and note the oxidizing agent used. This is the crucial first step in determining the reaction pathway.
2. Determine the Functional Group Present: Identify the functional group present in the substrate that is susceptible to oxidation. This could be an alcohol, aldehyde, ketone, or alkene.
3. Consider the Strength and Selectivity of the Oxidizing Agent: The oxidizing agent's strength determines the extent of oxidation. A strong oxidizing agent might completely oxidize a primary alcohol to a carboxylic acid, while a milder agent might stop at the aldehyde stage. Selectivity refers to the ability of an oxidizing agent to oxidize one functional group in the presence of others.
4. Apply the Oxidation Rules: Several rules govern the oxidation of different functional groups. These rules provide a framework for predicting the products:
-
Primary Alcohols: Strong oxidizing agents (like KMnO₄ or chromic acid) oxidize primary alcohols to carboxylic acids. Milder oxidizing agents (like PCC) oxidize them to aldehydes.
-
Secondary Alcohols: Both strong and mild oxidizing agents typically oxidize secondary alcohols to ketones.
-
Tertiary Alcohols: Tertiary alcohols are generally resistant to oxidation under typical conditions.
-
Aldehydes: Aldehydes are easily oxidized to carboxylic acids by strong oxidizing agents.
-
Ketones: Ketones are generally resistant to oxidation under typical conditions. However, under very harsh conditions, they can undergo oxidative cleavage.
-
Alkenes: Strong oxidizing agents like KMnO₄ can cleave alkenes, forming carboxylic acids or ketones depending on the substitution pattern of the double bond.
5. Draw the Product: Once you have determined the functional group transformation, carefully draw the structure of the oxidized product. Remember to account for any changes in the oxidation state of the carbon atoms involved.
Examples: Drawing Oxidation Products
Let's work through some specific examples to illustrate the process of drawing the correct organic product of an oxidation reaction.
Example 1: Oxidation of 1-propanol using chromic acid.
- Substrate: 1-propanol (a primary alcohol)
- Oxidizing Agent: Chromic acid (a strong oxidizing agent)
Since chromic acid is a strong oxidizing agent and 1-propanol is a primary alcohol, the product will be propanoic acid.
[Insert image showing 1-propanol being oxidized to propanoic acid by chromic acid]
Example 2: Oxidation of cyclohexanol using PCC.
- Substrate: Cyclohexanol (a secondary alcohol)
- Oxidizing Agent: PCC (a mild oxidizing agent)
PCC oxidizes secondary alcohols to ketones. Therefore, the product will be cyclohexanone.
[Insert image showing cyclohexanol being oxidized to cyclohexanone by PCC]
Example 3: Oxidation of 2-methyl-2-butanol with Jones reagent.
- Substrate: 2-methyl-2-butanol (a tertiary alcohol)
- Oxidizing Agent: Jones reagent (a strong oxidizing agent)
Tertiary alcohols are resistant to oxidation; therefore, no reaction will occur. The product remains 2-methyl-2-butanol.
[Insert image showing 2-methyl-2-butanol remaining unchanged after treatment with Jones reagent]
Example 4: Oxidation of benzaldehyde using KMnO₄.
- Substrate: Benzaldehyde (an aldehyde)
- Oxidizing Agent: KMnO₄ (a strong oxidizing agent)
KMnO₄ will oxidize benzaldehyde to benzoic acid.
[Insert image showing benzaldehyde being oxidized to benzoic acid by KMnO₄]
Example 5: Oxidation of 1-hexene using KMnO₄ in basic conditions.
- Substrate: 1-hexene (an alkene)
- Oxidizing Agent: KMnO₄ (a strong oxidizing agent) in basic conditions
KMnO₄ in basic conditions cleaves the double bond. The product will be hexanoic acid.
[Insert image showing 1-hexene being cleaved by KMnO₄ to form hexanoic acid]
Troubleshooting Common Mistakes
Even with a thorough understanding of the principles, predicting oxidation products can be challenging. Here are some common mistakes to avoid:
-
Incorrectly identifying the functional group: Double-check the substrate's structure and accurately identify the functional group susceptible to oxidation.
-
Overlooking the oxidizing agent's strength and selectivity: Remember that the choice of oxidizing agent significantly influences the reaction outcome. A strong oxidizing agent might lead to over-oxidation, while a milder one might result in incomplete oxidation.
-
Ignoring stereochemistry: In some cases, oxidation reactions can affect stereochemistry. Pay attention to the stereochemical implications of the reaction.
-
Not considering side reactions: Some oxidation reactions may undergo side reactions, leading to the formation of unexpected byproducts. Be aware of potential side reactions and consider their impact on the product distribution.
Advanced Oxidation Reactions
This guide has focused primarily on the basic oxidation reactions. More advanced oxidation reactions exist, involving more complex substrates, reagents, and reaction conditions. Further studies will expand your understanding of these reactions.
Conclusion
Mastering the art of predicting the products of oxidation reactions is crucial for success in organic chemistry. By following the steps outlined in this guide, carefully analyzing the substrate and oxidizing agent, and applying the appropriate oxidation rules, you can confidently draw the correct organic product. Remember to practice regularly and review the principles to reinforce your understanding. With consistent effort, you’ll develop the skills necessary to tackle more complex oxidation reactions with ease.
Latest Posts
Latest Posts
-
The Encoding Specificity Principle Is A Hypothesis That States
Mar 19, 2025
-
A Debit Balance In The Allowance For Doubtful Accounts
Mar 19, 2025
-
Select The Best Term For Each Definition Below
Mar 19, 2025
-
In Case Of An Emergency The Receptionist At Leading Edge
Mar 19, 2025
-
Which Of The Following Expressions Is Correct
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about Draw The Correct Organic Product Of The Oxidation Reaction Shown . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
