Write The Condensed Structure For Each Of These Skeletal Structures
Holbox
Mar 20, 2025 · 5 min read
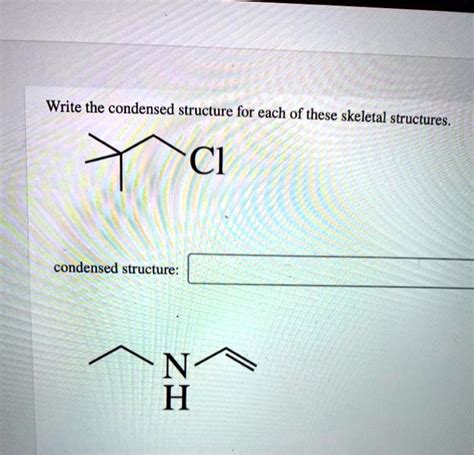
Table of Contents
Unveiling the Condensed Structural Formulas: A Comprehensive Guide
Understanding organic chemistry often hinges on efficiently representing complex molecules. While skeletal structures provide a visual representation of carbon-carbon bonds and attached functional groups, condensed structural formulas offer a more compact and readily interpretable textual format. This article delves deep into the art of converting skeletal structures into their condensed counterparts, exploring various examples and detailing the underlying principles.
From Skeletal Structures to Condensed Formulas: A Step-by-Step Approach
Skeletal structures, also known as line-angle formulas, utilize a simplified representation where carbon atoms are implied at the intersections and ends of lines. Hydrogen atoms bonded to carbons are usually omitted, and only other atoms (like oxygen, nitrogen, chlorine, etc.) are explicitly shown. Converting these to condensed formulas requires a systematic approach.
Key Principles:
- Identify the Carbon Backbone: Start by tracing the main carbon chain in the skeletal structure. This forms the foundation of your condensed formula.
- Determine Substituents: Identify any functional groups or atoms attached to the carbon backbone. These will be incorporated into the condensed formula.
- Order Matters: The order of atoms in the condensed formula reflects their arrangement in the skeletal structure. Branched structures need careful attention to placement.
- Parentheses for Branching: Use parentheses to denote branching groups, ensuring clarity and accuracy in the representation.
Let's illustrate this process with examples:
Example 1: A Simple Alkane
Let's consider a simple straight-chain alkane with five carbons:
CH3
|
CH3-CH-CH2-CH2-CH3
|
CH3
Condensed Formula: CH<sub>3</sub>CH(CH<sub>3</sub>)CH<sub>2</sub>CH<sub>2</sub>CH<sub>3</sub> or (CH<sub>3</sub>)<sub>2</sub>CHCH<sub>2</sub>CH<sub>2</sub>CH<sub>3</sub> (Both are acceptable)
Example 2: Introducing a Functional Group
Now let's add a functional group, say an alcohol (-OH), to the previous structure:
CH3
|
CH3-CH-CH2-CH(OH)-CH3
|
CH3
Condensed Formula: CH<sub>3</sub>CH(CH<sub>3</sub>)CH<sub>2</sub>CH(OH)CH<sub>3</sub>
Example 3: A More Complex Structure
Let's tackle a more intricate skeletal structure, incorporating a ring and multiple substituents:
CH3
|
CH3-C-CH2-CH3
|
CH2
|
CH2
/ \
CH2 CH3
Condensed Formula: CH<sub>3</sub>C(CH<sub>3</sub>)(CH<sub>2</sub>CH<sub>3</sub>)CH<sub>2</sub>CH<sub>2</sub>CH<sub>2</sub>CH<sub>3</sub>
Example 4: Aromatic Rings
Aromatic compounds, particularly benzene derivatives, require careful consideration. Benzene's ring structure is often simplified in skeletal form.
Benzene (C<sub>6</sub>H<sub>6</sub>):
/ \
/ \
/ \
\ /
\ /
\ /
Condensed Formula: C<sub>6</sub>H<sub>6</sub> (or you might see a benzene ring symbol within a condensed formula).
Toluene (methylbenzene):
CH3
|
/ \
/ \
/ \
\ /
\ /
\ /
Condensed Formula: C<sub>6</sub>H<sub>5</sub>CH<sub>3</sub>
Dealing with Multiple Substituents on Benzene:
When benzene has multiple substituents, their positions are crucial. Using numbers to indicate positions on the ring is common. For example, 1,3-dimethylbenzene (m-xylene) would be represented as:
1,3-Dimethylbenzene:
CH3
|
/ \
/ \
/ \
CH3
\ /
\ /
Condensed Formula: 1,3-(CH<sub>3</sub>)<sub>2</sub>C<sub>6</sub>H<sub>4</sub> or m-C<sub>6</sub>H<sub>4</sub>(CH<sub>3</sub>)<sub>2</sub>
Beyond the Basics: Advanced Considerations
As molecular complexity increases, so does the challenge of accurately representing them in condensed form. Here are some advanced considerations:
Dealing with Cyclic Structures
Condensed formulas for cyclic compounds are often more challenging. It's beneficial to systematically number the atoms in the ring and represent substituents using their positions.
Cyclohexane with a methyl group:
CH3
|
/ \
/ \
/ \
\ /
\ /
\ /
Condensed Formula: CH<sub>3</sub>C<sub>6</sub>H<sub>11</sub> (While this is correct, a more illustrative representation might include the position of the methyl group if it matters, such as 1-methylcyclohexane)
Stereochemistry and Isomerism
Condensed formulas typically do not convey stereochemical information (e.g., R/S configuration or cis/trans isomerism). For a complete representation, additional notations are necessary.
Polyfunctional Molecules
Molecules with multiple functional groups require a systematic approach to list each functional group in a concise yet clear manner, often specifying their locations within the molecule.
Practical Applications and Importance
The ability to readily interconvert skeletal and condensed structural formulas is a fundamental skill in organic chemistry. It's crucial for:
- Interpreting chemical data: Condensed formulas are prevalent in spectral data interpretation (NMR, IR, Mass Spec), making understanding these formulas essential for correlating experimental results with molecular structures.
- Naming organic compounds: Condensed formulas are used alongside IUPAC nomenclature to clearly identify and classify molecules.
- Predicting chemical properties: The condensed formula provides a succinct depiction of a molecule's composition, aiding in predicting its reactivity and other chemical properties.
- Designing organic syntheses: Condensed formulas simplify the representation of reactants and products during the planning and execution of organic syntheses.
Conclusion: Mastering Condensed Structural Formulas
The conversion of skeletal structures into condensed structural formulas is a crucial skill for anyone studying or working with organic molecules. Mastering this technique requires practice and attention to detail. By understanding the underlying principles and following a systematic approach, one can accurately represent even the most complex organic molecules in their compact and easily interpretable condensed form. This allows for efficient communication, analysis, and manipulation of molecular structures within the realm of organic chemistry. Remember, practice makes perfect! The more examples you work through, the more comfortable and confident you will become in translating between skeletal and condensed structures. This skill will serve as a powerful tool throughout your studies and career in the chemical sciences.
Latest Posts
Latest Posts
-
The Selected Inventory Costing Method Impacts
Mar 20, 2025
-
Regular Feelings Of Hunger Are An Example Of
Mar 20, 2025
-
The Term Capital Structure Refers To
Mar 20, 2025
-
To Increase The Money Supply The Federal Reserve Could
Mar 20, 2025
-
The Current Portion Of Long Term Debt Should
Mar 20, 2025
Related Post
Thank you for visiting our website which covers about Write The Condensed Structure For Each Of These Skeletal Structures . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
