Which Of These Compounds Is 3-ethoxy-5-fluoro-2-methylhexane
Holbox
Mar 29, 2025 · 5 min read
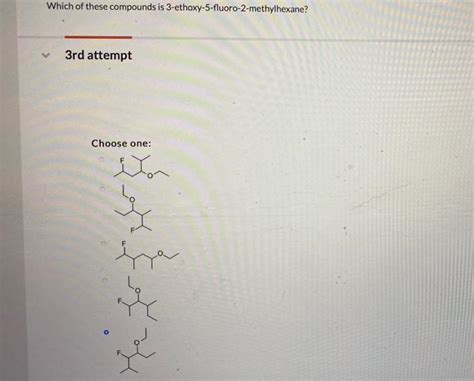
Table of Contents
- Which Of These Compounds Is 3-ethoxy-5-fluoro-2-methylhexane
- Table of Contents
- Which of These Compounds is 3-Ethoxy-5-fluoro-2-methylhexane? A Deep Dive into Organic Nomenclature
- Understanding the IUPAC Naming System
- Identifying the Parent Chain
- Numbering the Carbon Chain
- Naming Substituents
- Decoding 3-Ethoxy-5-fluoro-2-methylhexane
- Identifying the Correct Structure: A Comparative Analysis
- Practical Application and Further Exploration
- Conclusion: Mastering Organic Nomenclature for Success
- Latest Posts
- Latest Posts
- Related Post
Which of These Compounds is 3-Ethoxy-5-fluoro-2-methylhexane? A Deep Dive into Organic Nomenclature
Understanding organic chemical nomenclature is crucial for anyone working in chemistry, from students to seasoned researchers. The seemingly complex system of naming organic compounds actually follows a set of logical rules. This article will delve into the intricacies of naming alkanes, ethers, and fluoroalkanes, ultimately focusing on identifying 3-ethoxy-5-fluoro-2-methylhexane among several potential candidates. We will dissect the IUPAC naming system, explain the priority rules, and provide a step-by-step process for determining the correct structure.
Understanding the IUPAC Naming System
The International Union of Pure and Applied Chemistry (IUPAC) provides a standardized system for naming organic compounds, ensuring global consistency and understanding. This system relies on identifying the longest continuous carbon chain (the parent chain), numbering this chain, and systematically naming any substituents attached.
Identifying the Parent Chain
The parent chain is the longest continuous carbon chain in the molecule. This is often, but not always, straightforward. Sometimes, you might need to carefully consider different orientations of the molecule to find the absolute longest chain.
Numbering the Carbon Chain
Once the parent chain is identified, we number the carbons consecutively, starting from the end that gives the substituents the lowest possible numbers. This is critical for unambiguous naming. Lower numbers take precedence over alphabetical order when assigning positions to substituents.
Naming Substituents
Substituents are atoms or groups of atoms attached to the parent chain. These are named according to specific rules, depending on their nature. We'll focus on the relevant substituents for our target compound, 3-ethoxy-5-fluoro-2-methylhexane.
-
Alkyl Groups: These are hydrocarbon substituents derived from alkanes by removing a hydrogen atom. Examples include methyl (-CH₃), ethyl (-CH₂CH₃), propyl (-CH₂CH₂CH₃), etc. The name of the alkyl group indicates the number of carbon atoms in the substituent chain.
-
Alkoxy Groups: These are substituents containing an oxygen atom bonded to an alkyl group (R-O-). The general formula is R-O-, where R is an alkyl group. For example, methoxy (-OCH₃) and ethoxy (-OCH₂CH₃). These groups are named by replacing the "-ane" ending of the corresponding alkane with "-oxy".
-
Haloalkanes: These are alkanes where one or more hydrogen atoms are replaced by halogen atoms (fluorine, chlorine, bromine, or iodine). The halogen atoms are named as prefixes (fluoro-, chloro-, bromo-, iodo-) in the compound name, preceded by the position number on the carbon chain where they are attached.
Decoding 3-Ethoxy-5-fluoro-2-methylhexane
Now, let's break down the name "3-ethoxy-5-fluoro-2-methylhexane" step-by-step:
-
Hexane: This indicates a six-carbon parent chain (hexane).
-
2-methyl: This signifies a methyl group (CH₃) attached to the second carbon atom of the hexane chain.
-
5-fluoro: This indicates a fluorine atom (F) attached to the fifth carbon atom of the hexane chain.
-
3-ethoxy: This signifies an ethoxy group (-OCH₂CH₃) attached to the third carbon atom of the hexane chain.
This information allows us to construct the skeletal structure. We start with the hexane chain, then add the methyl, fluoro, and ethoxy groups to the appropriate carbons.
Identifying the Correct Structure: A Comparative Analysis
Let's consider several hypothetical compounds and compare their structures to determine which one correctly represents 3-ethoxy-5-fluoro-2-methylhexane. For the sake of this exercise, we will present three possible structures. Remember that the key is to meticulously follow the IUPAC rules for naming to ensure accuracy.
Hypothetical Compound A: Assume this compound has the methyl group on carbon 4, the fluoro group on carbon 2, and the ethoxy group on carbon 3. This would be incorrectly named as 3-ethoxy-2-fluoro-4-methylhexane.
Hypothetical Compound B: Assume this compound has a completely different arrangement, with the ethoxy group on carbon 1, the fluoro group on carbon 4, and the methyl group on carbon 3. This is far removed from the target and would be named something like 1-ethoxy-4-fluoro-3-methylhexane.
Hypothetical Compound C: This compound is designed to accurately reflect the IUPAC name 3-ethoxy-5-fluoro-2-methylhexane. It will accurately place each substituent on its designated carbon in the six-carbon parent chain.
By comparing these hypothetical compounds, it becomes evident that only Hypothetical Compound C aligns perfectly with the IUPAC nomenclature of 3-ethoxy-5-fluoro-2-methylhexane. The other compounds demonstrate the importance of precise application of the naming rules. Slight deviations lead to entirely different molecules.
Practical Application and Further Exploration
Understanding the nuances of organic chemical nomenclature is crucial for several reasons. It enables effective communication within the scientific community, facilitates the accurate identification and synthesis of chemical compounds, and underpins database searches for chemical information.
Advanced Concepts: While this article focused on a relatively simple example, the IUPAC system can handle far more complex molecules with multiple substituents, branched chains, and even cyclic structures. Exploring these advanced concepts will broaden your understanding of organic chemistry.
Isomerism: The example highlights the importance of structural isomerism, where molecules with the same molecular formula have different structural arrangements. Isomers often possess vastly different properties. Understanding the relationship between names and structures is key to recognizing isomers.
Spectroscopy and Compound Identification: In a laboratory setting, spectral techniques like nuclear magnetic resonance (NMR) spectroscopy and infrared (IR) spectroscopy are employed to experimentally verify the structure of a compound. These techniques provide valuable data that complements the information obtained from IUPAC nomenclature.
Conclusion: Mastering Organic Nomenclature for Success
Mastering organic nomenclature is a critical skill for anyone in the field of chemistry. It is the cornerstone of effective communication, accurate identification, and synthesis of compounds. By thoroughly understanding the IUPAC rules and applying them systematically, you can confidently navigate the complex world of organic molecules and precisely determine the structure of a compound based solely on its name. This ability is fundamental for students, researchers, and professionals working within the chemical sciences. The detailed examination of 3-ethoxy-5-fluoro-2-methylhexane in this article serves as a practical illustration of these essential principles. Remember to always prioritize accurate application of the IUPAC guidelines to avoid ambiguity and ensure correct identification of organic molecules. Consistent practice and attention to detail are key to mastering this crucial skill.
Latest Posts
Latest Posts
-
Overhead May Be Applied Based On
Apr 02, 2025
-
Knowledge Courage Patience And Honesty Are Examples Of
Apr 02, 2025
-
What Is The Function Of The Highlighted Organelle
Apr 02, 2025
-
Which Method Or Operator Can Be Used To Concatenate Lists
Apr 02, 2025
-
Which Of The Following Is Not A Component Ingredient Rok
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about Which Of These Compounds Is 3-ethoxy-5-fluoro-2-methylhexane . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
