Which Of The Following Is True Of Any S Enantiomer
Holbox
Mar 24, 2025 · 6 min read
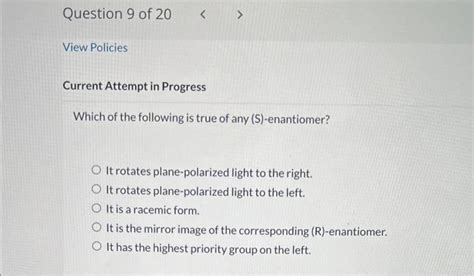
Table of Contents
- Which Of The Following Is True Of Any S Enantiomer
- Table of Contents
- Which of the Following is True of Any S Enantiomer? Understanding Chirality in Organic Chemistry
- Defining Enantiomers and the S Configuration
- Properties of S Enantiomers: What Makes Them Unique?
- Optical Activity: The Defining Difference
- Different Interactions with Chiral Environments: The Biological Significance
- Common Misconceptions about S Enantiomers
- Implications of Chirality and Enantiomer Separation
- Advanced Concepts and Further Exploration
- Conclusion: The Importance of Understanding S Enantiomers
- Latest Posts
- Latest Posts
- Related Post
Which of the Following is True of Any S Enantiomer? Understanding Chirality in Organic Chemistry
Understanding enantiomers is crucial in organic chemistry and related fields like pharmacology and biochemistry. This article delves deep into the properties and characteristics of S enantiomers, clarifying common misconceptions and highlighting their significance. We'll explore what defines an S enantiomer, its relationship to R enantiomers, and the implications of chirality in various applications.
Defining Enantiomers and the S Configuration
Enantiomers are a type of stereoisomer. Stereoisomers are molecules with the same molecular formula and connectivity but differ in the spatial arrangement of their atoms. Specifically, enantiomers are non-superimposable mirror images of each other. Think of your left and right hands – they're mirror images, but you can't perfectly overlay one onto the other. This non-superimposability is the defining characteristic of enantiomers.
The S and R configuration system, known as the Cahn-Ingold-Prelog (CIP) priority rules, is a standardized nomenclature system used to designate the absolute configuration of chiral centers. A chiral center (or stereocenter) is an atom, usually carbon, bonded to four different groups. The CIP rules assign priorities to the four groups based on atomic number. The higher the atomic number, the higher the priority.
To determine the S or R configuration:
-
Assign priorities: Assign priorities (1-4) to the four substituents attached to the chiral center, with 1 being the highest priority and 4 the lowest. This is based on atomic number; if the atoms directly attached are the same, consider the next atoms in the chain until a difference is found.
-
Orient the molecule: Orient the molecule so that the lowest priority group (4) points away from you. This is often done visually, imagining the molecule in 3D space.
-
Trace the path: Trace the path from the highest priority group (1) to the second-highest (2), then to the third-highest (3). If this path proceeds in a clockwise direction, the configuration is designated as R (from rectus, Latin for "right"). If the path is counterclockwise, the configuration is designated as S (sinister, Latin for "left").
Properties of S Enantiomers: What Makes Them Unique?
While S and R enantiomers possess identical physical properties like melting point, boiling point, and density, they differ significantly in their interaction with plane-polarized light and other chiral molecules.
Optical Activity: The Defining Difference
One of the most crucial differences lies in their optical activity. Enantiomers rotate the plane of plane-polarized light in opposite directions. An S enantiomer may rotate the plane of polarized light to the left (levo-rotatory, denoted as -), while its R enantiomer rotates it to the right (dextro-rotatory, denoted as +). The magnitude of rotation is identical, only the direction differs. This property is measured using a polarimeter.
It's crucial to understand that the S/R designation and the +/- (d/l) notation are independent. The absolute configuration (S or R) is determined by the CIP rules, while the sign of optical rotation (+ or -) is experimentally determined and does not directly correlate to the S/R assignment. A molecule can be S(+) or S(-), depending on its specific structure and interactions with polarized light.
Different Interactions with Chiral Environments: The Biological Significance
The interaction of enantiomers with other chiral molecules, especially biologically relevant molecules like enzymes and receptors, is fundamentally different. Enzymes, for instance, are often highly specific in their substrate binding. An enzyme that interacts with one enantiomer may not interact at all, or with significantly reduced affinity, with its counterpart. This principle is paramount in pharmacology.
Consider a drug molecule with a chiral center. One enantiomer (e.g., the S enantiomer) might be therapeutically active, while the other (R enantiomer) could be inactive or even toxic. This is because the active enantiomer fits perfectly into the receptor site, initiating a biological response. The inactive or toxic enantiomer, due to its different spatial arrangement, might not fit the receptor site or might bind to a different site, causing adverse effects. This necessitates careful consideration of enantiomeric purity in pharmaceutical drug development and production.
Common Misconceptions about S Enantiomers
Several misconceptions surround S enantiomers, which need clarification:
-
S enantiomers are always levorotatory (-): This is false. The S/R designation refers to absolute configuration, while (+) and (-) indicate the direction of optical rotation. A molecule can be S(+) or S(-).
-
S enantiomers are always more active than R enantiomers: This is also false. The biological activity depends on the specific molecule and its interaction with its biological target, not solely on its absolute configuration. One enantiomer may be more active, less active, or even toxic compared to its counterpart.
-
All chiral molecules have an S or R configuration: While the CIP rules apply to most chiral molecules, some molecules may possess multiple chiral centers, leading to more complex stereochemical descriptions. Furthermore, some molecules might exhibit other forms of stereoisomerism, like diastereomers.
Implications of Chirality and Enantiomer Separation
The significance of chirality extends across many fields. The ability to separate enantiomers, a process known as chiral resolution, is crucial in various industries, including:
-
Pharmaceutical Industry: Producing enantiomerically pure drugs ensures efficacy and minimizes adverse effects. The development of new chiral separation techniques is an active area of research.
-
Food Industry: The flavor and aroma of many food products depend on the presence of specific enantiomers. Chiral separation helps in enhancing or reducing specific flavors and odors.
-
Agricultural Chemistry: Pesticides and herbicides often exhibit different activities depending on their chirality. Using only the active enantiomer increases effectiveness and minimizes environmental impact.
Advanced Concepts and Further Exploration
For a more in-depth understanding of chirality and enantiomers, you can explore these advanced topics:
- Diastereomers: Stereoisomers that are not mirror images of each other.
- Meso compounds: Achiral molecules with chiral centers.
- Chiral Chromatography: Advanced techniques used for enantiomer separation.
- Conformational Analysis: Studying the different spatial arrangements of a molecule due to rotation around single bonds.
Conclusion: The Importance of Understanding S Enantiomers
In conclusion, understanding the properties and characteristics of S enantiomers is crucial in many scientific disciplines. The S designation denotes the absolute configuration of a chiral molecule determined by the Cahn-Ingold-Prelog rules. While the S/R configuration doesn't directly predict optical activity or biological activity, it provides a systematic way to describe the three-dimensional structure of chiral molecules. Recognizing that the biological activity of a molecule is heavily influenced by its chirality is paramount in areas like drug development and environmental science. The continuous exploration and advancement in techniques related to chiral resolution further highlight the pervasive importance of understanding this fundamental aspect of organic chemistry.
Latest Posts
Latest Posts
-
Draw The Main Lewis Structure Of Nof
Mar 26, 2025
-
All Of The Following Describe Blockchain
Mar 26, 2025
-
Physical Examination And Health Assessment Book
Mar 26, 2025
-
The Blood Platelet Counts Of A Group
Mar 26, 2025
-
Which Of The Following Research Designs Will Allow Cause And Effect Conclusions
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about Which Of The Following Is True Of Any S Enantiomer . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
