Which Of The Following Is An Oxidation Reduction Reaction
Holbox
Mar 29, 2025 · 6 min read
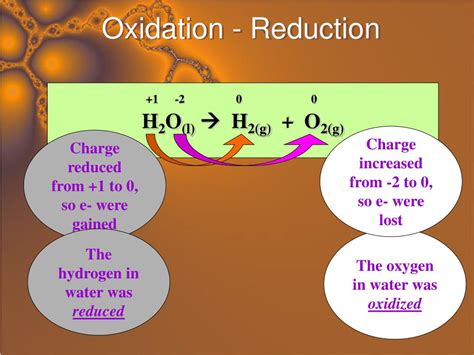
Table of Contents
- Which Of The Following Is An Oxidation Reduction Reaction
- Table of Contents
- Which of the Following is an Oxidation-Reduction Reaction? A Comprehensive Guide
- Understanding the Fundamentals of Redox Reactions
- Oxidation: The Loss of Electrons
- Reduction: The Gain of Electrons
- Identifying Redox Reactions: Key Indicators
- Distinguishing Redox Reactions from Other Reaction Types
- Examples and Practice Problems
- Advanced Concepts in Redox Reactions
- Conclusion
- Latest Posts
- Latest Posts
- Related Post
Which of the Following is an Oxidation-Reduction Reaction? A Comprehensive Guide
Oxidation-reduction reactions, also known as redox reactions, are fundamental chemical processes that involve the transfer of electrons between species. Understanding how to identify these reactions is crucial in various fields, from chemistry and biology to environmental science and materials engineering. This comprehensive guide will delve into the intricacies of redox reactions, providing you with the tools to confidently identify them in different chemical scenarios. We'll explore the core concepts, provide numerous examples, and offer strategies to distinguish redox reactions from other types of chemical transformations.
Understanding the Fundamentals of Redox Reactions
At the heart of every redox reaction lies the transfer of electrons. One species loses electrons (oxidation), while another gains electrons (reduction). These two processes are always coupled; you cannot have oxidation without reduction, and vice versa. This interconnectedness is represented by the term "redox," a portmanteau of "reduction" and "oxidation."
Oxidation: The Loss of Electrons
Oxidation is characterized by an increase in oxidation state. This means that an atom or ion loses electrons, becoming more positively charged or less negatively charged. Remember the mnemonic device OIL RIG – Oxidation Is Loss, Reduction Is Gain – to help you remember the electron transfer direction.
Examples of Oxidation:
- The rusting of iron: Iron (Fe) loses electrons to oxygen (O2) in the presence of water, forming iron oxide (rust, Fe2O3). Fe → Fe³⁺ + 3e⁻
- The combustion of methane: Methane (CH4) reacts with oxygen (O2) to produce carbon dioxide (CO2) and water (H2O). Carbon in methane is oxidized from an oxidation state of -4 to +4 in carbon dioxide.
- The reaction of magnesium with hydrochloric acid: Magnesium (Mg) loses electrons to hydrogen ions (H⁺) to form magnesium ions (Mg²⁺) and hydrogen gas (H2).
Reduction: The Gain of Electrons
Reduction is characterized by a decrease in oxidation state. This means that an atom or ion gains electrons, becoming more negatively charged or less positively charged.
Examples of Reduction:
- The formation of silver from silver ions: Silver ions (Ag⁺) gain electrons to form solid silver (Ag). Ag⁺ + e⁻ → Ag
- The conversion of oxygen to water: Oxygen (O2) gains electrons during the combustion of fuels, forming water (H2O). Oxygen's oxidation state changes from 0 to -2.
- The reduction of copper(II) ions: Copper(II) ions (Cu²⁺) gain electrons to form copper metal (Cu). Cu²⁺ + 2e⁻ → Cu
Identifying Redox Reactions: Key Indicators
Several key indicators can help you determine whether a given reaction is a redox reaction:
- Changes in Oxidation States: The most definitive way to identify a redox reaction is by tracking the changes in oxidation states of the atoms involved. If one or more atoms increase in oxidation state (oxidation) while others decrease (reduction), it's a redox reaction.
- Presence of Oxidizing and Reducing Agents: Redox reactions always involve two reactants: an oxidizing agent (the species that gets reduced, accepting electrons) and a reducing agent (the species that gets oxidized, donating electrons). Identifying these agents is strong evidence for a redox reaction.
- Electron Transfer: Explicitly showing the transfer of electrons in the balanced chemical equation is a clear indication of a redox reaction. Half-reactions, which separate the oxidation and reduction processes, are a useful tool for this.
- Change in Color: Some redox reactions are accompanied by a visible color change, indicating a change in the oxidation state of one or more species. This is not always reliable, as many reactions don't involve color changes.
Distinguishing Redox Reactions from Other Reaction Types
It's crucial to distinguish redox reactions from other types of chemical reactions, such as acid-base reactions and precipitation reactions.
Acid-Base Reactions: These involve the transfer of protons (H⁺ ions) rather than electrons. No changes in oxidation states occur.
Precipitation Reactions: These involve the formation of an insoluble solid (precipitate) from the reaction of two soluble ionic compounds. Again, no electron transfer is involved, and oxidation states remain unchanged.
Examples and Practice Problems
Let's analyze some examples to solidify your understanding:
Example 1:
2Fe(s) + 3Cl₂(g) → 2FeCl₃(s)
- Iron (Fe): Oxidation state changes from 0 to +3 (oxidation)
- Chlorine (Cl): Oxidation state changes from 0 to -1 (reduction)
- Conclusion: This is a redox reaction; iron is oxidized, and chlorine is reduced.
Example 2:
HCl(aq) + NaOH(aq) → NaCl(aq) + H₂O(l)
- Analysis: This is an acid-base neutralization reaction. No changes in oxidation states occur. It's not a redox reaction.
Example 3:
2H₂(g) + O₂(g) → 2H₂O(l)
- Hydrogen (H): Oxidation state changes from 0 to +1 (oxidation)
- Oxygen (O): Oxidation state changes from 0 to -2 (reduction)
- Conclusion: This is a redox reaction; hydrogen is oxidized, and oxygen is reduced. This is a classic combustion reaction, a type of redox reaction.
Example 4:
AgNO₃(aq) + NaCl(aq) → AgCl(s) + NaNO₃(aq)
- Analysis: This is a precipitation reaction. No changes in oxidation states occur. It is not a redox reaction.
Practice Problem 1:
Determine if the following reaction is a redox reaction:
Cu(s) + 2AgNO₃(aq) → 2Ag(s) + Cu(NO₃)₂(aq)
(Solution: Yes. Cu is oxidized, and Ag⁺ is reduced.)
Practice Problem 2:
Is the following reaction a redox reaction?
H₂SO₄(aq) + 2KOH(aq) → K₂SO₄(aq) + 2H₂O(l)
(Solution: No. This is an acid-base neutralization reaction. There is no change in oxidation states.)
Advanced Concepts in Redox Reactions
Beyond the basic principles, several advanced concepts enhance our understanding of redox reactions:
- Balancing Redox Reactions: Balancing redox equations often requires more complex techniques than simple balancing. The half-reaction method, involving separating oxidation and reduction half-reactions, is a common approach.
- Electrochemistry: Redox reactions are fundamental to electrochemistry, the study of the relationship between chemical reactions and electrical energy. Examples include batteries and fuel cells.
- Redox Titrations: These are analytical techniques used to determine the concentration of an unknown substance by reacting it with a solution of known concentration (a titrant) in a redox reaction.
- Biological Redox Reactions: Redox reactions are essential in biological systems, playing crucial roles in metabolism, respiration, and photosynthesis.
Conclusion
Identifying oxidation-reduction reactions is a critical skill in chemistry and related disciplines. By understanding the fundamental principles of electron transfer, oxidation states, and the key indicators of redox reactions, you can confidently analyze chemical equations and determine whether a reaction involves the transfer of electrons. Remember the mnemonic OIL RIG, practice identifying oxidizing and reducing agents, and master the techniques for balancing redox equations to become proficient in identifying and understanding these crucial chemical processes. This comprehensive guide has provided you with a robust foundation; continue exploring this fascinating area of chemistry to deepen your understanding and mastery.
Latest Posts
Latest Posts
-
Experiment 10 Analysis Of An Aluminum Zinc Alloy
Apr 02, 2025
-
Correctly Label The Following Anatomical Parts Of A Kidney
Apr 02, 2025
-
Identify The Possible Double Jeopardy For Elderly Ethnic Minority Individuals
Apr 02, 2025
-
How Many Moles Are In 15 Grams Of Lithium
Apr 02, 2025
-
Humans Carry A Variety Of Non Functional Genetic Sequences Called
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about Which Of The Following Is An Oxidation Reduction Reaction . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
