Which Of The Following Best Explains Diffusion
Holbox
Mar 24, 2025 · 8 min read
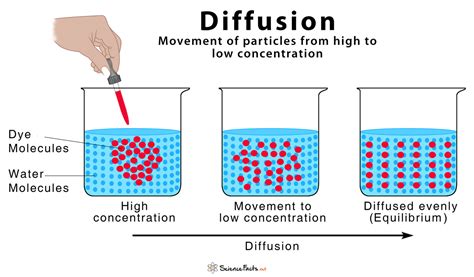
Table of Contents
- Which Of The Following Best Explains Diffusion
- Table of Contents
- Which of the Following Best Explains Diffusion? A Deep Dive into the Process
- Defining Diffusion: More Than Just Spreading Out
- Mechanisms of Diffusion: How Do Particles Move?
- Diffusion in Gases: A Free-for-All
- Diffusion in Liquids: A More Crowded Space
- Diffusion in Solids: A Struggle Through the Lattice
- Factors Affecting the Rate of Diffusion: Speeding Up or Slowing Down
- 1. Concentration Gradient: The Steeper, the Faster
- 2. Temperature: Heat Things Up
- 3. Mass of the Particles: Heavier is Slower
- 4. Medium of Diffusion: The Path Matters
- 5. Surface Area: More Space, Faster Diffusion
- 6. Distance: The Longer the Journey, the Slower the Diffusion
- Types of Diffusion: Beyond the Basics
- 1. Simple Diffusion: Unassisted Movement
- 2. Facilitated Diffusion: A Helping Hand
- 3. Osmosis: Water's Special Case
- Diffusion in Different Contexts: Applications Across Disciplines
- 1. Biology: Essential for Life
- 2. Chemistry: Reactions and Transport
- 3. Materials Science: Designing New Materials
- 4. Social Sciences: The Spread of Ideas
- Conclusion: Diffusion – A Ubiquitous and Vital Process
- Latest Posts
- Latest Posts
- Related Post
Which of the Following Best Explains Diffusion? A Deep Dive into the Process
Diffusion, a seemingly simple process, plays a crucial role in numerous scientific fields, from biology and chemistry to materials science and even social sciences. Understanding diffusion is key to comprehending how substances move, how materials react, and how societal trends spread. But what exactly is diffusion, and which explanation best captures its essence? This article will explore various definitions, delve into the mechanisms behind diffusion, and clarify the factors that influence its rate.
Defining Diffusion: More Than Just Spreading Out
The most straightforward definition of diffusion is the net movement of particles from a region of higher concentration to a region of lower concentration. This movement continues until the particles are evenly distributed throughout the available space. Think of a drop of food coloring added to a glass of water. Initially, the coloring is concentrated in one spot, but over time, it spreads out, eventually coloring the entire glass uniformly. This is diffusion in action.
However, this simple definition, while correct, doesn't fully capture the complexity of the process. It doesn't explicitly mention the driving force behind diffusion, nor does it address the different types of diffusion or the factors influencing its speed. Therefore, a more comprehensive definition should incorporate these elements:
A more complete definition: Diffusion is a passive transport process driven by the random motion of particles, leading to the net movement of particles from a region of high chemical potential to a region of low chemical potential until equilibrium is reached. This process occurs across various mediums, including gases, liquids, and solids, and is influenced by factors such as temperature, concentration gradient, and the nature of the medium itself.
This expanded definition highlights several key aspects:
- Passive Transport: Diffusion doesn't require energy input. It's a spontaneous process driven by the inherent kinetic energy of the particles.
- Random Motion: The particles aren't moving in a coordinated fashion; their movement is random and chaotic. However, the net effect of this random movement is a directional flow from high to low concentration.
- Chemical Potential: This is a more precise measure than simply "concentration." Chemical potential accounts for factors like temperature and pressure that also influence particle movement. At equilibrium, the chemical potential is equal throughout the system.
Mechanisms of Diffusion: How Do Particles Move?
The mechanism of diffusion is intrinsically linked to the kinetic theory of matter. Particles, whether atoms, molecules, or ions, are constantly in motion, possessing kinetic energy. This motion is random; particles collide with each other and with the surrounding medium. The higher the temperature, the greater the kinetic energy and the faster the particles move, leading to faster diffusion.
The specific mechanism varies depending on the state of matter:
Diffusion in Gases: A Free-for-All
In gases, particles are far apart and experience minimal intermolecular forces. Their movement is essentially unrestricted, leading to relatively rapid diffusion. The mean free path (average distance a particle travels between collisions) is relatively long.
Diffusion in Liquids: A More Crowded Space
Diffusion in liquids is slower than in gases because the particles are closer together and experience stronger intermolecular forces. This restricts their movement, and the mean free path is shorter. However, particles still have sufficient kinetic energy to overcome these forces and move randomly.
Diffusion in Solids: A Struggle Through the Lattice
Diffusion in solids is the slowest because particles are tightly packed in a crystal lattice. Their movement is significantly restricted, and they can only diffuse by migrating through interstitial spaces or by exchanging positions with other particles in the lattice. This process is highly dependent on the temperature and the structure of the solid. Higher temperatures provide the necessary energy for atoms to overcome the energy barrier for diffusion.
Factors Affecting the Rate of Diffusion: Speeding Up or Slowing Down
Several factors influence how quickly diffusion occurs:
1. Concentration Gradient: The Steeper, the Faster
The concentration gradient, the difference in concentration between two regions, is directly proportional to the rate of diffusion. A steeper gradient (larger difference in concentration) leads to faster diffusion. This is because the driving force for diffusion is greater when there's a larger difference in concentration.
2. Temperature: Heat Things Up
Temperature significantly affects the rate of diffusion. Higher temperatures increase the kinetic energy of particles, leading to faster movement and thus faster diffusion. Conversely, lower temperatures slow down diffusion.
3. Mass of the Particles: Heavier is Slower
The mass of the particles also plays a role. Heavier particles move more slowly than lighter particles, resulting in slower diffusion rates for heavier particles.
4. Medium of Diffusion: The Path Matters
The medium through which diffusion occurs significantly impacts its rate. Diffusion is faster in gases than in liquids, and fastest in gases with low molecular weight. Diffusion in solids is the slowest, being highly dependent on the crystal structure and any imperfections within the lattice.
5. Surface Area: More Space, Faster Diffusion
The surface area available for diffusion also influences the rate. A larger surface area allows for more particles to participate in the diffusion process simultaneously, leading to faster diffusion.
6. Distance: The Longer the Journey, the Slower the Diffusion
The distance over which diffusion must occur is inversely proportional to the rate. The longer the distance, the slower the diffusion. This is because particles need more time to travel the greater distance.
Types of Diffusion: Beyond the Basics
While the basic concept of diffusion remains consistent, there are various types and nuances to consider:
1. Simple Diffusion: Unassisted Movement
Simple diffusion is the most basic type, where particles move directly across a membrane or through a medium without any assistance from carrier proteins or other transport mechanisms. This is the type of diffusion described in the earlier sections.
2. Facilitated Diffusion: A Helping Hand
Facilitated diffusion involves the movement of particles across a membrane with the help of membrane proteins. These proteins act as channels or carriers, facilitating the passage of specific molecules that otherwise wouldn't be able to cross the membrane easily. While still passive, facilitated diffusion is more specific and often faster than simple diffusion.
3. Osmosis: Water's Special Case
Osmosis is a specific type of diffusion involving the movement of water molecules across a selectively permeable membrane from a region of high water concentration (low solute concentration) to a region of low water concentration (high solute concentration). This process is vital for maintaining the water balance in cells and organisms.
Diffusion in Different Contexts: Applications Across Disciplines
The importance of diffusion extends far beyond the classroom. It's a fundamental process with wide-ranging applications:
1. Biology: Essential for Life
In biology, diffusion is essential for numerous processes:
- Gas exchange in lungs: Oxygen diffuses from the lungs into the blood, and carbon dioxide diffuses from the blood into the lungs.
- Nutrient absorption in the intestines: Nutrients diffuse from the intestines into the bloodstream.
- Waste removal in the kidneys: Waste products diffuse from the blood into the kidneys.
- Neurotransmission: Neurotransmitters diffuse across synapses to transmit signals between neurons.
2. Chemistry: Reactions and Transport
In chemistry, diffusion is crucial for chemical reactions to occur. Reactants must diffuse together before they can interact and form products. Diffusion also plays a role in separating mixtures and controlling reaction rates.
3. Materials Science: Designing New Materials
In materials science, understanding diffusion is essential for designing new materials with specific properties. For example, diffusion is used in the manufacturing of alloys, where different metals are mixed at high temperatures to create materials with improved strength and other desirable properties. Diffusion bonding is a solid-state welding technique that relies on atomic diffusion at elevated temperatures to achieve a metallurgical bond between two surfaces.
4. Social Sciences: The Spread of Ideas
Diffusion isn't limited to the physical world. In social sciences, the concept of diffusion is used to describe the spread of ideas, innovations, and cultural practices within a population. The rate of diffusion in this context is affected by factors such as communication networks, social norms, and the perceived value of the innovation.
Conclusion: Diffusion – A Ubiquitous and Vital Process
In summary, diffusion is a fundamental process driven by the random motion of particles, leading to the net movement of particles from a region of high chemical potential to a region of low chemical potential until equilibrium is reached. Understanding the mechanisms and factors influencing diffusion is crucial in various scientific disciplines. While a simple definition captures the essence of diffusion, a more comprehensive definition highlights the passive nature of the process, the role of chemical potential, and the various factors influencing its rate. The importance of diffusion extends from the molecular level to societal dynamics, demonstrating its ubiquitous and vital role in the world around us. Whether it's gas exchange in our lungs, the manufacture of advanced materials, or the spread of innovations throughout society, diffusion remains a central and fascinating process worth continued investigation.
Latest Posts
Latest Posts
-
How Can Dna Methylation Silence Transcription
Mar 29, 2025
-
What Is The Haploid Number Of Chromosomes For A Cat
Mar 29, 2025
-
Which Image Highlights The Parent Chain
Mar 29, 2025
-
Use Your Molecular Modeling Kit To Create A Cho2 Ion
Mar 29, 2025
-
According To Globalization Supporters What Is An Advantage Of Globalization
Mar 29, 2025
Related Post
Thank you for visiting our website which covers about Which Of The Following Best Explains Diffusion . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
