What Makes Agglutination By Antibodies Possible
Holbox
Mar 26, 2025 · 5 min read
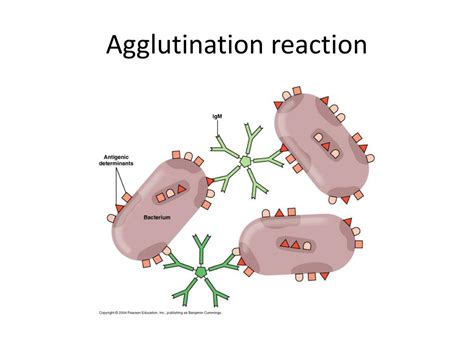
Table of Contents
- What Makes Agglutination By Antibodies Possible
- Table of Contents
- What Makes Agglutination by Antibodies Possible?
- The Role of Antibodies in Agglutination
- The Multivalency of Antigens and Antibodies
- The Forces Driving Agglutination
- 1. Non-covalent Interactions: The Glue of Agglutination
- 2. The Role of Electrostatic Forces
- 3. The Importance of Antibody Isotype
- Factors Affecting Agglutination
- Applications of Agglutination
- Beyond Basic Agglutination: Enhanced Techniques
- Conclusion
- Latest Posts
- Latest Posts
- Related Post
What Makes Agglutination by Antibodies Possible?
Agglutination, the visible clumping of particles, is a crucial process in various biological and medical contexts. It's particularly important in immunology, where it's a hallmark of antibody-mediated immune responses. Understanding the mechanisms behind antibody-mediated agglutination is key to appreciating the power and precision of our immune systems and developing diagnostic and therapeutic tools. This article will delve deep into the intricacies of this process, exploring the molecular interactions, influencing factors, and applications of agglutination.
The Role of Antibodies in Agglutination
Antibodies, also known as immunoglobulins (Ig), are Y-shaped glycoproteins produced by plasma B cells. They play a vital role in the adaptive immune system, specifically in recognizing and neutralizing foreign substances called antigens. These antigens can be anything from bacteria and viruses to toxins and allergens. The specificity of antibody-antigen binding lies at the heart of agglutination.
Each antibody molecule possesses two identical antigen-binding sites, located at the tips of the Y-shape's arms. These sites, also known as paratopes, are highly specific for a particular epitope, a specific region on the antigen. This remarkable specificity is the result of a precise three-dimensional arrangement of amino acid residues within the paratope, creating a lock-and-key interaction with its corresponding epitope.
The Multivalency of Antigens and Antibodies
A key factor enabling agglutination is the multivalency of both antigens and antibodies. Multivalency refers to the presence of multiple identical epitopes on a single antigen molecule (or particle carrying antigens) and multiple antigen-binding sites on a single antibody molecule. This allows a single antibody to bind to multiple antigen particles simultaneously, forming a bridge between them.
Imagine a scenario where a bacterium is coated with many copies of the same antigen. Several antibodies can then bind to different epitopes on this bacterium. Each antibody, in turn, can bind to another bacterium carrying the same antigen. This chain reaction leads to the formation of large aggregates – the visible clumps characteristic of agglutination. This process is greatly enhanced by the flexibility of the antibody molecule, allowing it to reach across to different antigen particles, maximizing the efficiency of cross-linking.
The Forces Driving Agglutination
The formation of antibody-antigen complexes leading to agglutination isn't solely dependent on specific binding. Various forces contribute to the stability and efficiency of this process:
1. Non-covalent Interactions: The Glue of Agglutination
The initial interaction between the antibody paratope and the antigen epitope is primarily driven by non-covalent bonds. These include:
- Hydrogen bonds: Weak bonds formed between hydrogen atoms and electronegative atoms like oxygen or nitrogen.
- Ionic bonds: Electrostatic attractions between oppositely charged groups.
- Hydrophobic interactions: Interactions between nonpolar regions, driven by the tendency to minimize contact with water.
- Van der Waals forces: Weak, short-range attractive forces between molecules.
While individually weak, the cumulative effect of many such bonds creates a strong and specific antibody-antigen interaction. The strength of these interactions (affinity) directly influences the efficiency of agglutination. Higher affinity leads to more stable complexes and more extensive clumping.
2. The Role of Electrostatic Forces
The net surface charge of both antibodies and antigens plays a significant role. The electrostatic repulsion between similarly charged particles can hinder agglutination. Conversely, optimizing conditions to reduce repulsive forces, for example by adjusting the ionic strength of the solution, can enhance agglutination.
3. The Importance of Antibody Isotype
Different classes of immunoglobulins (IgM, IgG, IgA, IgD, IgE) exhibit different agglutinating capabilities. IgM, with its pentameric structure (five antibody monomers joined together), is particularly effective at agglutination due to its high valency (10 antigen-binding sites). This allows it to bridge multiple antigen particles efficiently, leading to rapid and extensive clumping. IgG, being a monomer, is less efficient in agglutination but still contributes significantly.
Factors Affecting Agglutination
Several factors influence the efficiency of antibody-mediated agglutination:
- Antibody concentration: Higher antibody concentrations generally lead to more extensive agglutination, up to a point. Very high concentrations can sometimes lead to prozone phenomenon, where excess antibody hinders cross-linking.
- Antigen concentration: Similar to antibody concentration, an optimal antigen concentration is needed. Too little antigen might lead to insufficient cross-linking, while too much can also interfere with the process.
- Temperature: Optimal temperature enhances antibody-antigen binding and therefore agglutination. Temperature too high might denature the proteins, while low temperatures might slow down the reaction rate.
- pH: The pH of the solution can affect the electrostatic interactions between antibodies and antigens and consequently affect agglutination.
- Ionic strength: As mentioned, the ionic strength of the solution can influence electrostatic repulsion and consequently affect agglutination.
Applications of Agglutination
Agglutination reactions are widely used in various diagnostic and therapeutic applications:
- Blood typing: Determining blood groups relies on agglutination reactions between blood antigens and corresponding antibodies.
- Disease diagnosis: Agglutination tests are used to detect various infectious agents like bacteria and viruses. Examples include Widal test for typhoid fever and rapid diagnostic tests for influenza.
- Pregnancy tests: Home pregnancy tests utilize agglutination to detect human chorionic gonadotropin (hCG) in urine.
- Therapeutic applications: Agglutination can be used to remove unwanted cells or substances from the body, for instance in targeted drug delivery.
Beyond Basic Agglutination: Enhanced Techniques
Several techniques enhance the visualization and sensitivity of agglutination reactions:
- Coombs test (antiglobulin test): This test detects antibodies bound to red blood cells that are not causing visible agglutination.
- Passive agglutination: This involves coating inert particles (e.g., latex beads) with antigens, increasing the visibility of the reaction.
- Hemagglutination inhibition: This technique is used to detect the presence of specific antibodies by their ability to inhibit viral hemagglutination.
Conclusion
Antibody-mediated agglutination is a complex process governed by a delicate interplay of specific antibody-antigen binding and various physical forces. The multivalency of both antibodies and antigens, alongside non-covalent interactions, electrostatic forces, and antibody isotype, dictates the efficiency of this vital immunological process. Understanding these mechanisms is crucial for developing and interpreting various diagnostic and therapeutic tools that leverage the power of agglutination in medicine and beyond. Further research into optimizing agglutination reactions could lead to improved diagnostic accuracy and more effective therapeutic strategies. The field continues to evolve, constantly revealing new insights into the intricacies of this fundamental biological phenomenon.
Latest Posts
Latest Posts
-
Art Labeling Activity Levels Of Protein Structure
Mar 27, 2025
-
According To The Cognitive View Of Classical Conditioning
Mar 27, 2025
-
Acting As A Mentor Gives Managers
Mar 27, 2025
-
Motion In Two Dimensions Mech Hw 21
Mar 27, 2025
-
A Decrease In Demand While Holding Supply Constant Results In
Mar 27, 2025
Related Post
Thank you for visiting our website which covers about What Makes Agglutination By Antibodies Possible . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
