What Is The Relationship Between The Following Two Compounds
Holbox
Mar 17, 2025 · 6 min read
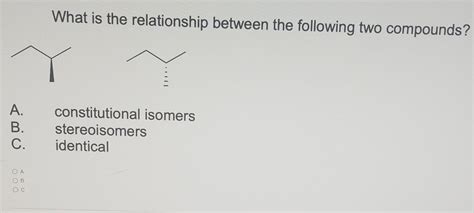
Table of Contents
- What Is The Relationship Between The Following Two Compounds
- Table of Contents
- Unveiling the Intertwined Relationship Between Aspirin and Salicylic Acid: From Willow Bark to Modern Medicine
- The Ancient Roots: Salicylic Acid from Willow Bark
- The Synthesis of Aspirin: A Tale of Chemical Modification
- The Chemistry Connection: A Subtle but Significant Difference
- The Mechanism of Action: Targeting Inflammation and Pain
- Therapeutic Applications: Beyond Pain Relief
- The Future of Salicylic Acid and Aspirin Derivatives
- Conclusion: A Legacy of Pain Relief
- Latest Posts
- Latest Posts
- Related Post
Unveiling the Intertwined Relationship Between Aspirin and Salicylic Acid: From Willow Bark to Modern Medicine
The seemingly simple relationship between aspirin and salicylic acid belies a fascinating journey through history, chemistry, and medicine. While often perceived as distinct entities, these two compounds share a profound connection, one built on a foundation of natural origins and a shared mechanism of action. Understanding this relationship offers a deeper appreciation for the evolution of pain relief and the ingenuity of pharmaceutical development.
The Ancient Roots: Salicylic Acid from Willow Bark
Long before the advent of modern medicine, civilizations across the globe recognized the analgesic and anti-inflammatory properties of willow bark. Ancient Egyptian, Mesopotamian, and Greek texts make reference to willow bark extracts being used to alleviate pain and fever. The active component responsible for these therapeutic effects, however, remained unknown for centuries.
It wasn't until the 19th century that scientists began to isolate and identify the key ingredient. In 1828, Italian chemist Raffaele Piria successfully isolated salicylic acid from salicin, a glycoside found abundantly in willow bark. This marked a pivotal moment, transforming an age-old folk remedy into a subject of scientific inquiry. Salicylic acid itself, however, possessed some drawbacks. Its acidic nature often caused gastrointestinal irritation, limiting its widespread therapeutic use.
The Synthesis of Aspirin: A Tale of Chemical Modification
The quest for a more tolerable yet equally effective pain reliever led to the synthesis of acetylsalicylic acid, better known as aspirin. In 1897, German chemist Felix Hoffmann, working for the Bayer company, synthesized aspirin by acetylating salicylic acid. This seemingly simple chemical modification proved to be revolutionary.
The acetylation process reduced the acidity of salicylic acid, significantly mitigating its harsh effects on the stomach lining. The resulting compound, aspirin, retained the potent analgesic and anti-inflammatory properties of its precursor while exhibiting vastly improved tolerability. This discovery marked a turning point in the history of pain management, paving the way for the mass production and widespread use of aspirin as a safe and effective over-the-counter medication.
The Chemistry Connection: A Subtle but Significant Difference
Chemically, the relationship between aspirin and salicylic acid is straightforward: aspirin is simply the acetylated derivative of salicylic acid. This means that an acetyl group (CH₃CO) is attached to the hydroxyl (-OH) group of the salicylic acid molecule. This seemingly minor alteration, however, has profound consequences for the compound's properties.
Salicylic acid:
- Contains a free phenolic hydroxyl group (-OH) that contributes to its acidic nature and potential for gastrointestinal irritation.
- Possesses strong anti-inflammatory, analgesic, and antipyretic (fever-reducing) properties.
Aspirin (Acetylsalicylic Acid):
- The acetylation of the hydroxyl group masks the acidity, leading to improved gastrointestinal tolerance.
- Retains the potent anti-inflammatory, analgesic, and antipyretic properties of salicylic acid, albeit often with a slightly milder effect.
- Undergoes hydrolysis in the body, converting back to salicylic acid, which then exerts its therapeutic effects. This explains why both compounds ultimately achieve similar physiological outcomes.
This subtle chemical difference explains why aspirin is generally better tolerated than salicylic acid despite sharing a similar mechanism of action. The acetylation protects the stomach lining from the harsh effects of the free hydroxyl group, allowing for safer and more convenient administration.
The Mechanism of Action: Targeting Inflammation and Pain
Both salicylic acid and aspirin exert their therapeutic effects by inhibiting the production of prostaglandins. Prostaglandins are lipid compounds that play a crucial role in mediating inflammation, pain, and fever. They are produced by an enzyme called cyclooxygenase (COX).
COX enzymes exist in two main isoforms: COX-1 and COX-2. COX-1 is constitutively expressed, meaning it's always present in the body and plays a role in maintaining the integrity of the gastrointestinal tract and protecting against ulcers. COX-2, on the other hand, is induced in response to inflammation and injury.
Both salicylic acid and aspirin act as non-selective COX inhibitors, meaning they inhibit both COX-1 and COX-2 enzymes. This inhibition reduces prostaglandin production, leading to the reduction of inflammation, pain, and fever. However, the inhibition of COX-1 is responsible for the gastrointestinal side effects associated with both compounds. Aspirin's acetylation offers some degree of reduced COX-1 inhibition compared to salicylic acid, thus explaining the improved gastrointestinal tolerance.
The development of selective COX-2 inhibitors (e.g., celecoxib, rofecoxib) aimed to address the gastrointestinal side effects associated with non-selective COX inhibitors. These newer drugs selectively inhibit COX-2, reducing inflammation and pain while minimizing the gastrointestinal risks. However, concerns regarding cardiovascular side effects led to the withdrawal or restricted use of some COX-2 inhibitors.
Therapeutic Applications: Beyond Pain Relief
While both salicylic acid and aspirin are primarily known for their analgesic and anti-inflammatory properties, their applications extend beyond pain relief. Salicylic acid, in particular, finds widespread use in topical preparations for treating various skin conditions:
- Acne: Salicylic acid effectively exfoliates the skin, removing dead skin cells and unclogging pores.
- Psoriasis: Its anti-inflammatory properties help to manage the symptoms of psoriasis, a chronic skin condition characterized by inflamed, scaly patches.
- Warts: Salicylic acid can be used to treat warts by gradually dissolving the affected tissue.
Aspirin, on the other hand, also enjoys a broader spectrum of therapeutic applications:
- Prevention of Cardiovascular Disease: Aspirin is commonly prescribed at low doses to prevent blood clots, reducing the risk of heart attacks and strokes. This effect is attributed to its ability to inhibit platelet aggregation (blood clotting).
- Treatment of Kawasaki Disease: Aspirin plays a critical role in managing Kawasaki disease, a condition that causes inflammation in the blood vessels.
The Future of Salicylic Acid and Aspirin Derivatives
The journey from willow bark to modern medicine underscores the continuous evolution of drug discovery and development. While both salicylic acid and aspirin have firmly established their place in the pharmacopoeia, research continues to explore their therapeutic potential and investigate novel derivatives with enhanced efficacy and safety profiles. The understanding of the intricate relationship between these two compounds is pivotal for continuing this important work.
Conclusion: A Legacy of Pain Relief
The relationship between salicylic acid and aspirin is one of remarkable synergy. Starting from the natural extract of willow bark, the isolation of salicylic acid laid the foundation for the synthesis of aspirin – a safer and more effective derivative that revolutionized pain management and continues to impact global health. Their shared mechanism of action, centered on the inhibition of prostaglandin synthesis, underscores the enduring relevance of these compounds in treating inflammation, pain, and a range of other medical conditions. Their legacy extends beyond simple pain relief, highlighting the power of natural products, chemical ingenuity, and the continuous quest for improved therapeutic interventions. The story of salicylic acid and aspirin remains a testament to the ongoing evolution of medicine, driven by the persistent pursuit of better health and well-being.
Latest Posts
Latest Posts
-
What Is The Correct Structure Of Aspirin
Mar 18, 2025
-
Which Of The Following Should Appear In Every Ad
Mar 18, 2025
-
Label The Photomicrograph Of Compact Bone
Mar 18, 2025
-
Which Of The Following Is True Concerning Hero Images
Mar 18, 2025
-
S Express Your Answer As An Ion
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about What Is The Relationship Between The Following Two Compounds . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
