What Is The Correct Structure Of Aspirin
Holbox
Mar 18, 2025 · 6 min read
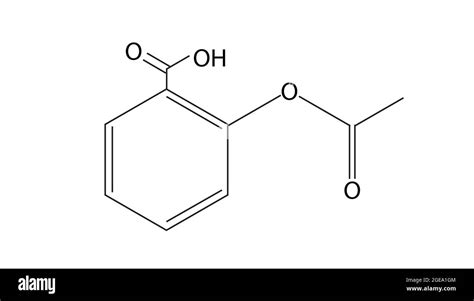
Table of Contents
What is the Correct Structure of Aspirin? A Deep Dive into its Chemistry and Pharmacology
Aspirin, a household name synonymous with pain relief and fever reduction, boasts a deceptively simple yet fascinating chemical structure. Understanding this structure is key to understanding its mechanism of action, its efficacy, and its potential side effects. This in-depth article delves into the intricacies of aspirin's structure, exploring its composition, properties, and the relationship between its molecular architecture and its pharmacological effects.
The Chemical Identity: Acetylsalicylic Acid
Aspirin's chemical name is acetylsalicylic acid. This name itself provides clues to its structure. Let's break it down:
-
Acetyl: This prefix indicates the presence of an acetyl group, a crucial functional group with the formula CH₃CO-. This group is attached to the rest of the molecule and plays a vital role in aspirin's activity.
-
Salicylic Acid: This part of the name reveals the core structure of the molecule. Salicylic acid itself is a naturally occurring compound found in willow bark and has analgesic and anti-inflammatory properties. However, salicylic acid can be irritating to the stomach.
Therefore, aspirin is essentially a modified version of salicylic acid, with an acetyl group added. This modification significantly alters its properties, making it a more effective and better-tolerated drug.
Deconstructing the Molecular Structure
Aspirin's structure can be represented in several ways, including:
-
Chemical Formula: C₉H₈O₄
-
Structural Formula: This shows the arrangement of atoms and bonds within the molecule. You'll often see it depicted as a benzene ring (a six-carbon ring with alternating single and double bonds) with various functional groups attached.
-
Benzene Ring: This forms the core of the salicylic acid portion. It's a stable, aromatic ring structure.
-
Carboxylic Acid Group (-COOH): This is a key functional group responsible for aspirin's acidic properties. It's located on one of the carbons of the benzene ring. It is this group that contributes to the low pH of aspirin solutions.
-
Ester Group (-COO-): This crucial group links the acetyl group to the salicylic acid portion. This ester linkage is formed during the synthesis of aspirin and is vital for its pharmacological action. The ester group is the result of the acetylation of salicylic acid.
-
Acetyl Group (-COCH₃): This is attached to the hydroxyl group (-OH) of the salicylic acid via the ester bond. This acetyl group's addition is what differentiates aspirin from salicylic acid and fundamentally alters its properties.
-
-
3D Model: A three-dimensional model provides the most accurate representation of the molecule, showcasing the spatial arrangement of atoms and their bonds. This illustrates the molecule's shape and how it might interact with receptors in the body.
The Importance of the Ester Linkage
The ester linkage connecting the acetyl group and the salicylic acid moiety is absolutely critical to aspirin's function. Here's why:
-
Pharmacokinetic Properties: The ester linkage affects how aspirin is absorbed, distributed, metabolized, and excreted (ADME) in the body. The relatively non-polar nature of the ester enhances its absorption in the gastrointestinal tract.
-
Mechanism of Action: Aspirin's primary pharmacological effect stems from its ability to inhibit cyclooxygenase (COX) enzymes. Once inside the body, the ester bond is hydrolyzed (broken down by water), releasing salicylic acid. It is the salicylic acid that then inhibits COX enzymes.
-
Reduced Gastric Irritation: While salicylic acid itself can irritate the stomach lining, the acetylation protects against this irritation to a significant degree. However, even aspirin can cause gastrointestinal distress in some individuals.
Synthesis of Aspirin: From Salicylic Acid to the Active Compound
Aspirin is synthesized through a relatively simple chemical reaction known as acetylation. This process involves reacting salicylic acid with acetic anhydride in the presence of an acid catalyst, typically sulfuric acid or phosphoric acid. The reaction yields acetylsalicylic acid (aspirin) and acetic acid as a byproduct.
Aspirin's Mechanism of Action: COX Inhibition
The main mechanism of action involves the inhibition of cyclooxygenase (COX) enzymes. These enzymes are crucial in the biosynthesis of prostaglandins, thromboxanes, and prostacyclins – signaling molecules involved in pain, inflammation, and blood clotting.
COX-1 and COX-2 Isozymes: Distinct Roles and Selective Inhibition
There are two main isoforms of COX: COX-1 and COX-2.
-
COX-1: This enzyme plays a crucial role in maintaining the integrity of the gastrointestinal tract and platelet function. Non-selective NSAIDs, including aspirin, can inhibit COX-1, leading to potential side effects like gastrointestinal bleeding and increased bleeding risk.
-
COX-2: This enzyme is primarily induced during inflammation. COX-2 inhibitors are often preferred due to a reduced risk of gastrointestinal side effects. However, some COX-2 inhibitors have been linked to cardiovascular risks.
Aspirin's ability to inhibit both COX-1 and COX-2 enzymes contributes to its analgesic (pain-relieving), antipyretic (fever-reducing), and anti-inflammatory properties. However, its irreversible inhibition of COX-1 is also responsible for some of its side effects.
Clinical Applications of Aspirin: A Versatile Drug
Aspirin's multifaceted effects have resulted in a wide range of clinical applications:
-
Analgesic: Relieves mild to moderate pain, such as headaches, muscle aches, and menstrual cramps.
-
Antipyretic: Reduces fever associated with various illnesses.
-
Anti-inflammatory: Alleviates inflammation in conditions like arthritis.
-
Antiplatelet: This is arguably aspirin's most significant application. By irreversibly inhibiting COX-1 in platelets, aspirin prevents platelet aggregation (clumping), thereby reducing the risk of blood clots. This is crucial in preventing heart attacks, strokes, and other cardiovascular events. This is why low doses of aspirin are often prescribed for individuals at risk of cardiovascular disease.
Side Effects and Precautions: Understanding the Risks
While aspirin is generally safe when used as directed, potential side effects include:
-
Gastrointestinal Problems: Nausea, vomiting, heartburn, stomach ulcers, and gastrointestinal bleeding.
-
Allergic Reactions: Rashes, itching, swelling, and in rare cases, anaphylaxis.
-
Reye's Syndrome: A rare but serious condition affecting children and teenagers, characterized by brain swelling and liver damage. Aspirin should never be given to children or teenagers with viral infections.
-
Bleeding: Increased risk of bleeding due to the antiplatelet effect.
Conclusion: Aspirin – A Simple Structure, Complex Effects
The seemingly simple structure of aspirin belies its profound impact on medicine and healthcare. Understanding the chemical details – the benzene ring, the carboxylic acid group, the crucial ester linkage, and the acetyl group – is essential to comprehending its mechanism of action, its therapeutic applications, and its potential side effects. Its effectiveness in pain relief, fever reduction, and particularly its antiplatelet action makes it a cornerstone of modern medicine, while the importance of understanding its potential risks remains paramount for safe and effective use. Future research continues to explore aspirin's full potential and to develop safer and more effective aspirin-based therapies.
Latest Posts
Latest Posts
-
Record The Entry To Close The Dividends Account
Mar 18, 2025
-
Which Of The Following Best Describes The Operational Period Briefing
Mar 18, 2025
-
The Second Largest Number Of Pacs Are Those Associated With
Mar 18, 2025
-
The Accompanying Graph Represents Haydens Fro Yo
Mar 18, 2025
-
According To The Text Which Is True Of Leadership
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about What Is The Correct Structure Of Aspirin . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
