What Element Is Designated By The Orbital Diagram Below
Holbox
Mar 26, 2025 · 4 min read
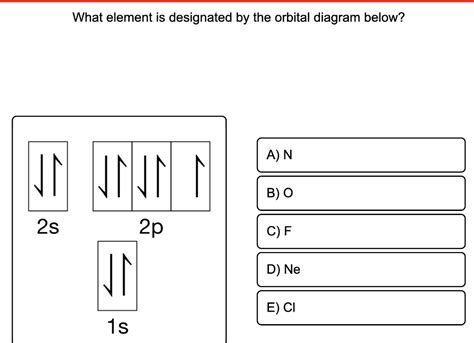
Table of Contents
What Element is Designated by the Orbital Diagram Below? A Deep Dive into Electron Configuration and Atomic Structure
Understanding electron configuration is fundamental to comprehending the behavior of elements and their place within the periodic table. Orbital diagrams, a visual representation of electron arrangement within an atom's shells and subshells, provide a powerful tool for this understanding. This article will delve into the interpretation of orbital diagrams, explain the rules governing electron placement, and demonstrate how to identify the element represented by a given diagram. We'll also explore the connection between electron configuration, atomic properties, and the periodic table's structure.
Understanding Orbital Diagrams
An orbital diagram is a pictorial representation of the arrangement of electrons within an atom's orbitals. Each orbital, a region of space where an electron is most likely to be found, can hold a maximum of two electrons, according to the Pauli Exclusion Principle. These electrons must have opposite spins, typically represented by arrows pointing up (↑) and down (↓). Orbitals are grouped into subshells (s, p, d, and f), which are further grouped into electron shells (energy levels) denoted by the principal quantum number (n = 1, 2, 3...).
Rules Governing Electron Configuration
Several key rules govern how electrons fill orbitals:
-
Aufbau Principle: Electrons fill orbitals starting with the lowest energy level and progressing to higher energy levels. The order of filling is generally: 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d, 7p... Note that there are exceptions to this rule, particularly with transition metals.
-
Hund's Rule: Within a subshell, electrons will individually occupy each orbital before pairing up in any one orbital. This minimizes electron-electron repulsion. This means each orbital within a subshell gets one electron before any orbital gets a second.
-
Pauli Exclusion Principle: No two electrons in an atom can have the same four quantum numbers (principal quantum number, azimuthal quantum number, magnetic quantum number, and spin quantum number). This means each orbital can hold a maximum of two electrons with opposite spins.
Interpreting an Example Orbital Diagram
Let's consider a hypothetical orbital diagram:
1s: ↑↓
2s: ↑↓
2p: ↑↓ ↑↓ ↑
3s: ↑↓
3p: ↑↓ ↑↓ ↑
Step-by-step Analysis:
-
Count the total number of electrons: Add up the electrons in each orbital: 2 + 2 + 3 + 2 + 3 = 12 electrons.
-
Determine the atomic number: The atomic number of an element is equal to the number of protons in its nucleus and also equal to the number of electrons in a neutral atom. Therefore, this atom has an atomic number of 12.
-
Identify the element: Using the periodic table, find the element with atomic number 12. This is Magnesium (Mg).
Advanced Scenarios and Exceptions
While the Aufbau principle provides a general guideline, exceptions exist, primarily among transition metals and lanthanides/actinides. These exceptions arise due to the relatively small energy differences between certain orbitals. For instance, chromium (Cr) and copper (Cu) have electron configurations that deviate from the predicted Aufbau order. This is due to the increased stability achieved by having half-filled or completely filled d subshells.
Consider the following orbital diagram:
1s: ↑↓
2s: ↑↓
2p: ↑↓ ↑↓ ↑↓
3s: ↑↓
3p: ↑↓ ↑↓ ↑↓
4s: ↑↓
3d: ↑ ↑ ↑ ↑ ↑
This shows a total of 24 electrons. Using the periodic table, we identify this as Chromium (Cr), demonstrating the exception to the Aufbau principle. Note that while the 4s orbital fills before the 3d, the final configuration places one electron in each 3d orbital before pairing, showcasing Hund's rule.
Orbital Diagrams and Periodic Trends
Orbital diagrams are closely related to periodic trends. The outermost electrons, known as valence electrons, are primarily responsible for an element's chemical properties and reactivity. Elements in the same group (vertical column) of the periodic table have similar valence electron configurations, leading to similar chemical behavior. For instance, all alkali metals (Group 1) have one valence electron in an s orbital, explaining their high reactivity.
Applying the Knowledge: Practice Examples
Let's work through a few more examples to solidify our understanding.
Example 1:
1s: ↑↓
2s: ↑↓
2p: ↑↓ ↑↓ ↑↓
3s: ↑↓
3p: ↑↓ ↑↓
This configuration represents 16 electrons. This corresponds to Sulfur (S).
Example 2:
1s: ↑↓
2s: ↑↓
2p: ↑↓ ↑↓ ↑
This configuration shows 7 electrons. This is Nitrogen (N).
Example 3 (a more challenging example with a transition metal):
1s: ↑↓
2s: ↑↓
2p: ↑↓ ↑↓ ↑↓
3s: ↑↓
3p: ↑↓ ↑↓ ↑↓
4s: ↑↓
3d: ↑ ↑ ↑ ↑ ↑
This shows 24 electrons, indicating Chromium (Cr), highlighting the exception to the Aufbau principle.
Conclusion
Understanding how to interpret orbital diagrams is crucial for understanding the fundamental principles of chemistry. By applying the Aufbau principle, Hund's rule, and the Pauli exclusion principle, we can accurately determine the electron configuration and identify the element represented by a given orbital diagram. Mastering this skill unlocks a deeper understanding of atomic structure, periodic trends, and chemical reactivity. Remember to account for exceptions to the Aufbau principle, especially when dealing with transition metals and inner transition elements. With practice, you'll become proficient in deciphering these diagrams and connecting them to the fascinating world of elements and their properties. Further exploration into quantum numbers and the wave-particle duality of electrons can provide an even more profound understanding of these fundamental concepts.
Latest Posts
Latest Posts
-
Two Disks Are Rotating About The Same Axis
Mar 30, 2025
-
A 5 Month Pregnant Patient Arrives In The Emergency Department
Mar 30, 2025
-
Which Two Items Are Closely Related To The Reserve Requirement
Mar 30, 2025
-
Use The Table To Answer The Questions
Mar 30, 2025
-
What Multiplies To 12 And Adds To
Mar 30, 2025
Related Post
Thank you for visiting our website which covers about What Element Is Designated By The Orbital Diagram Below . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
