Use Retrosynthetic Analysis To Suggest A Way
Holbox
Mar 18, 2025 · 5 min read
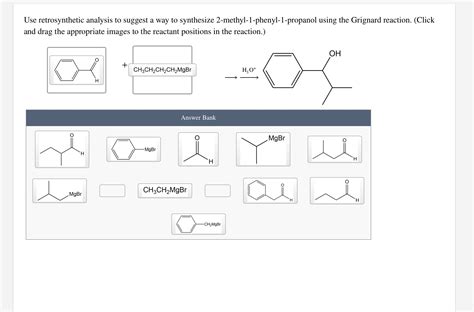
Table of Contents
Using Retrosynthetic Analysis to Suggest a Synthetic Route: A Comprehensive Guide
Retrosynthetic analysis, a powerful tool in organic chemistry, allows chemists to devise efficient synthetic routes for complex molecules. Instead of working forwards from simple starting materials, retrosynthetic analysis works backward from the target molecule, systematically disconnecting bonds to arrive at simpler precursors. This process, often described as "working backwards from the product," is crucial for designing efficient and practical syntheses, especially for intricate molecules. This comprehensive guide will delve into the principles and applications of retrosynthetic analysis, providing practical examples and insights to aid in your understanding.
Understanding the Fundamentals of Retrosynthetic Analysis
The core concept behind retrosynthetic analysis revolves around identifying disconnections. These are strategic bond cleavages that transform the target molecule into simpler, more readily available precursors or synthons. Each disconnection represents a retrosynthetic step, moving closer to commercially available starting materials. The process continues iteratively until simple and easily accessible building blocks are identified.
Key Principles and Strategies:
-
Functional Group Interconversions (FGI): This is a central concept. Recognizing how functional groups can be transformed into others is essential. For example, a ketone can be reduced to an alcohol, an alcohol can be oxidized to a ketone or aldehyde, and so on. This understanding guides the disconnection strategy.
-
Strategic Bond Disconnections: Not all bonds are created equal. Prioritizing bonds based on their reactivity and synthetic accessibility is critical. Common disconnections include those involving carbon-carbon double bonds (C=C), carbonyl groups (C=O), and carbon-heteroatom bonds (C-O, C-N, C-X where X is a halogen).
-
Synthons and Synthetic Equivalents: Synthons are idealized fragments that represent reactive intermediates. They aren't necessarily isolable but serve as conceptual tools. Synthetic equivalents are real, readily available reagents that mimic the reactivity of a synthon. For example, a Grignard reagent (RMgX) can serve as a synthetic equivalent for a carbanion synthon (R⁻).
-
Protecting Groups: Protecting groups are crucial for managing the reactivity of specific functional groups during the synthesis. They temporarily block unwanted reactions while allowing other transformations to occur. The choice of protecting group depends on the specific functional group and the reaction conditions.
-
Stereochemistry: Careful consideration of stereochemistry is paramount. Retrosynthetic analysis needs to account for stereoselective reactions to ensure the desired stereochemistry in the final product.
Practical Application of Retrosynthetic Analysis: Case Studies
Let's examine several examples to illustrate the application of retrosynthetic analysis. We'll focus on the thought process and strategy involved in designing synthetic routes, rather than providing detailed reaction mechanisms.
Example 1: Synthesis of 2-Methyl-2-butanol
Target Molecule: 2-Methyl-2-butanol
Retrosynthetic Analysis:
-
Disconnection 1: The C-O bond of the alcohol can be disconnected. This suggests a carbonyl compound (a ketone) as a precursor.
-
Disconnection 2: The ketone can be further disconnected into a Grignard reagent and an alkyl halide. A suitable Grignard reagent would be methylmagnesium bromide (CH₃MgBr) and the alkyl halide would be 2-bromobutane.
Forward Synthesis:
-
Formation of the Grignard reagent from bromomethane.
-
Reaction of the Grignard reagent with 2-bromobutane, followed by an acidic workup, yielding 2-methyl-2-butanol.
This example illustrates a simple, two-step synthesis derived through retrosynthetic reasoning.
Example 2: Synthesis of a More Complex Molecule - A β-Keto Ester
Target Molecule: Ethyl 3-oxobutanoate (ethyl acetoacetate)
Retrosynthetic Analysis:
-
Disconnection 1: The β-keto ester can be disconnected at the β-carbon-carbonyl bond. This suggests an ester and a ketone fragment.
-
Disconnection 2: The ketone fragment could be derived from an acid chloride and a nucleophile such as an ester enolate.
Forward Synthesis (Claisen Condensation):
-
Deprotonation of ethyl acetate to form an enolate.
-
Reaction of the enolate with ethyl acetate (another molecule) leads to a β-keto ester (ethyl 3-oxobutanoate) after acid workup.
This showcases the application of retrosynthetic analysis to a reaction that involves enolate chemistry.
Example 3: A Molecule Requiring Multiple Steps and Protecting Groups
Consider the synthesis of a molecule with multiple functional groups, potentially requiring protecting group strategies. Let's imagine a synthesis with an alcohol, ketone, and an amine. The exact retrosynthetic analysis would depend on the specific molecule and its functional group arrangement. However, the process would involve the following considerations:
-
Identifying Key Disconnections: Bonds near the most reactive functional groups (usually carbonyl groups) would be prioritized for disconnection.
-
Protecting Groups: If reactions on one functional group might affect another, protecting groups would be strategically incorporated into the retrosynthetic analysis. For instance, the alcohol might need protection before reactions involving the ketone or amine.
-
Multiple Synthetic Steps: The retrosynthetic analysis would likely involve several steps, moving from the target molecule to progressively simpler intermediates.
-
Reagent Selection: The choice of reagents would be guided by the compatibility with the protecting groups and the desired transformations. Careful consideration of selectivity is essential.
Advanced Techniques and Considerations
Several advanced strategies and considerations further refine the process of retrosynthetic analysis.
-
Computer-Assisted Retrosynthetic Analysis: Software programs and databases aid in identifying potential synthetic routes, accelerating the process. These programs incorporate large databases of known reactions and functional group transformations.
-
Combinatorial Chemistry: This approach explores a wide range of reactants and conditions to discover optimal synthetic paths.
-
Green Chemistry Principles: The design of synthetic routes should consider environmental impact. Minimizing waste, using safer reagents, and selecting energy-efficient reactions are crucial factors.
Conclusion
Retrosynthetic analysis is an indispensable tool in organic chemistry, streamlining the design of efficient and practical syntheses. By working backward from the target molecule, chemists can systematically identify suitable starting materials and reaction pathways. While simple examples demonstrate fundamental principles, the true power of retrosynthetic analysis lies in its application to complex molecules, requiring strategic disconnections, protecting groups, and a deep understanding of reaction mechanisms. The application of advanced techniques and considerations, such as computer assistance and green chemistry principles, further enhance the efficiency and sustainability of synthetic route design. Mastering retrosynthetic analysis is an essential skill for any aspiring organic chemist.
Latest Posts
Latest Posts
-
What Is The Product Of This Reaction
Mar 18, 2025
-
Record The Entry To Close The Dividends Account
Mar 18, 2025
-
Which Of The Following Best Describes The Operational Period Briefing
Mar 18, 2025
-
The Second Largest Number Of Pacs Are Those Associated With
Mar 18, 2025
-
The Accompanying Graph Represents Haydens Fro Yo
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about Use Retrosynthetic Analysis To Suggest A Way . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
