What Is The Product Of This Reaction
Holbox
Mar 18, 2025 · 6 min read
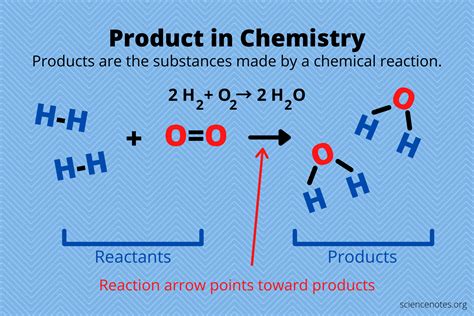
Table of Contents
- What Is The Product Of This Reaction
- Table of Contents
- What is the Product of This Reaction? A Deep Dive into Predicting Reaction Outcomes
- Understanding Reaction Mechanisms: The Key to Prediction
- Factors Influencing Reaction Outcomes: Beyond the Mechanism
- Predicting Products: A Step-by-Step Approach
- Examples of Reaction Predictions:
- Conclusion: Mastering the Art of Reaction Prediction
- Latest Posts
- Latest Posts
- Related Post
What is the Product of This Reaction? A Deep Dive into Predicting Reaction Outcomes
Predicting the product of a chemical reaction is a cornerstone of chemistry. It's not just about memorizing reactions; it requires understanding fundamental principles like reaction mechanisms, functional group transformations, and reaction kinetics. This article explores the multifaceted approach to determining the product of a chemical reaction, addressing various reaction types and the factors influencing their outcomes. We'll delve into strategies for predicting products, emphasizing the importance of considering reaction conditions, reagents, and the inherent reactivity of the starting materials.
Understanding Reaction Mechanisms: The Key to Prediction
Before we dive into specific examples, let's establish the importance of understanding reaction mechanisms. A reaction mechanism is a step-by-step description of how a reaction proceeds, detailing the bond breaking and bond formation processes involved. Knowing the mechanism allows us to predict not only the major product but also potential side products and byproducts. Different reaction mechanisms lead to different products, even with the same starting materials.
Examples of Common Reaction Mechanisms:
-
SN1 (Substitution Nucleophilic Unimolecular): This mechanism involves a two-step process: formation of a carbocation intermediate followed by nucleophilic attack. The stability of the carbocation intermediate heavily influences the product distribution. More stable carbocations (tertiary > secondary > primary) are formed preferentially. Rearrangements are also common in SN1 reactions.
-
SN2 (Substitution Nucleophilic Bimolecular): This is a concerted, one-step mechanism where the nucleophile attacks the substrate from the backside, leading to inversion of configuration. Steric hindrance significantly impacts SN2 reactions; bulky substrates react slower or not at all.
-
E1 (Elimination Unimolecular): Similar to SN1, E1 reactions involve a carbocation intermediate. However, instead of nucleophilic attack, a base abstracts a proton, leading to the formation of a double bond. The more substituted alkene is generally the major product (Zaitsev's rule).
-
E2 (Elimination Bimolecular): This is a concerted, one-step mechanism where the base abstracts a proton and the leaving group departs simultaneously, resulting in the formation of a double bond. Steric factors and the strength of the base influence the regioselectivity and stereoselectivity of E2 reactions.
-
Addition Reactions: These reactions involve the addition of a reagent across a multiple bond (double or triple bond). The regioselectivity and stereoselectivity of addition reactions depend on the nature of the reagent and the substrate. Markovnikov's rule predicts the regioselectivity of electrophilic addition to unsymmetrical alkenes.
-
Condensation Reactions: These reactions involve the combination of two molecules with the elimination of a small molecule, such as water or an alcohol. Examples include esterification, aldol condensation, and Claisen condensation.
Factors Influencing Reaction Outcomes: Beyond the Mechanism
While understanding the reaction mechanism is crucial, several other factors significantly influence the product formed:
-
Reactant Structure: The functional groups present, the steric hindrance, and the electronic effects within the reactants profoundly affect reactivity and product formation. Electron-donating groups can activate certain positions for reactions, while electron-withdrawing groups can deactivate them.
-
Reaction Conditions: Temperature, solvent, concentration, and pressure all play a critical role. High temperatures often favor faster reactions and sometimes lead to different product distributions due to changes in reaction pathways. The solvent can affect the solubility of reactants and intermediates, influencing reaction rates and selectivity.
-
Reagents: The choice of reagents (nucleophiles, electrophiles, bases, acids, catalysts) is paramount. Different reagents can lead to entirely different reaction pathways and products. For example, using a strong base in an elimination reaction might favor the less substituted alkene, whereas a weaker base might favor the more substituted alkene.
-
Catalysis: Catalysts can accelerate reactions and increase selectivity toward a specific product by providing an alternative, lower-energy reaction pathway. Enzymes are biological catalysts that exhibit remarkable specificity and selectivity.
-
Equilibria: Many reactions are reversible, existing in equilibrium between reactants and products. The equilibrium constant (K) determines the relative amounts of reactants and products at equilibrium. Manipulating reaction conditions (e.g., Le Chatelier's principle) can shift the equilibrium to favor the desired product.
Predicting Products: A Step-by-Step Approach
To predict the product of a reaction, follow these steps:
-
Identify the Functional Groups: Determine the functional groups present in the starting materials and reagents. This provides clues about the potential reaction pathways.
-
Determine the Reaction Type: Based on the functional groups and reagents, classify the reaction type (e.g., SN1, SN2, E1, E2, addition, condensation).
-
Consider the Reaction Mechanism: Understanding the mechanism helps predict the intermediate(s) and the final product(s). Draw out the mechanism step-by-step.
-
Analyze Stereochemistry: If applicable, consider the stereochemistry of the reactants and products. SN2 reactions, for example, proceed with inversion of configuration, while SN1 reactions often lead to racemization.
-
Assess Regioselectivity and Stereoselectivity: Determine which product(s) are formed preferentially based on factors like Markovnikov's rule (for addition reactions) or Zaitsev's rule (for elimination reactions).
-
Account for Side Reactions: Consider the possibility of side reactions and byproducts. These often arise due to competing reaction pathways.
-
Evaluate Reaction Conditions: The reaction conditions play a crucial role in influencing product formation. Consider the effects of temperature, solvent, and concentration.
-
Consider Catalysis: If a catalyst is used, understand its role in affecting the reaction mechanism and product distribution.
Examples of Reaction Predictions:
Let's illustrate with a couple of examples:
Example 1: Reaction of 2-bromobutane with potassium tert-butoxide (t-BuOK)
-
Functional Groups: 2-bromobutane (alkyl halide), potassium tert-butoxide (strong base)
-
Reaction Type: Elimination reaction (E2) due to the presence of a strong base and a good leaving group.
-
Mechanism: The tert-butoxide ion acts as a strong base, abstracting a proton from the β-carbon. Simultaneously, the bromide ion leaves, forming a double bond.
-
Product: The major product will be 2-butene (the more substituted alkene, following Zaitsev's rule). A minor amount of 1-butene might also be formed.
Example 2: Reaction of 2-methyl-2-propanol with concentrated sulfuric acid
-
Functional Groups: 2-methyl-2-propanol (tertiary alcohol), concentrated sulfuric acid (strong acid)
-
Reaction Type: Dehydration reaction (E1) due to the tertiary alcohol and the acidic conditions.
-
Mechanism: The sulfuric acid protonates the hydroxyl group, forming a good leaving group (water). A carbocation intermediate is formed, which then loses a proton to yield an alkene.
-
Product: The major product will be 2-methylpropene, the only possible alkene product in this case.
Conclusion: Mastering the Art of Reaction Prediction
Predicting the product of a chemical reaction requires a deep understanding of reaction mechanisms, functional group transformations, and the influence of reaction conditions. By systematically analyzing the reactants, reagents, and reaction conditions, coupled with a strong grasp of organic chemistry principles, we can reliably predict the major product(s) and anticipate potential side reactions. The examples provided in this article serve as a starting point for developing the necessary skills to accurately predict reaction outcomes, essential for success in organic chemistry and related fields. Consistent practice and thorough analysis of various reaction scenarios are key to honing these skills. Continuous learning and exploration of advanced concepts, such as pericyclic reactions and organometallic chemistry, will further enhance your ability to predict and understand complex reaction pathways.
Latest Posts
Latest Posts
-
Blake Eats Two Bags Of Potato Chips
Mar 19, 2025
-
The Core Element Of Any Term Is Its
Mar 19, 2025
-
The Demand For Grape Flavored Hubba Bubba Bubble Gum Is Likely
Mar 19, 2025
-
Apply The Comma Style Number Format To Range E3 E11
Mar 19, 2025
-
Business Level Strategies Are Concerned Specifically With
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about What Is The Product Of This Reaction . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
