Two Compounds A And B Have The Formula
Holbox
Mar 20, 2025 · 6 min read
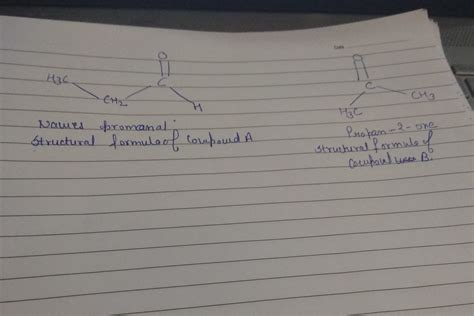
Table of Contents
Two Compounds A and B Have the Formula: Unveiling Isomerism and its Implications
The statement "Two compounds A and B have the formula..." immediately points towards the fascinating world of isomerism in chemistry. Isomers are molecules that share the same molecular formula but differ in their structural arrangement. This seemingly subtle difference can lead to vastly different physical and chemical properties. This article will delve deep into the potential scenarios when two compounds, A and B, share a formula, exploring various types of isomerism, their identification techniques, and the significance of this phenomenon in different fields.
Understanding Isomerism: A Foundation
Before we dive into specific examples, let's establish a solid understanding of isomerism. The fundamental concept rests on the fact that atoms within a molecule can arrange themselves in multiple ways, leading to different structures. This structural variation results in diverse properties, influencing everything from reactivity to melting and boiling points.
There are several major categories of isomerism:
1. Constitutional Isomerism (Structural Isomerism): This is the most fundamental type, where isomers differ in the connectivity of their atoms. This means that the atoms are bonded in different sequences or arrangements. Constitutional isomers can be further categorized into:
-
Chain Isomerism: This occurs when the carbon skeleton differs. For example, butane (CH<sub>3</sub>CH<sub>2</sub>CH<sub>2</sub>CH<sub>3</sub>) and methylpropane (CH<sub>3</sub>CH(CH<sub>3</sub>)CH<sub>3</sub>) are chain isomers. They both have the formula C<sub>4</sub>H<sub>10</sub>, but the arrangement of carbon atoms is different.
-
Position Isomerism: Here, the position of a functional group or substituent on the carbon chain varies. For instance, 1-chloropropane (ClCH<sub>2</sub>CH<sub>2</sub>CH<sub>3</sub>) and 2-chloropropane (CH<sub>3</sub>CHClCH<sub>3</sub>) are position isomers. Both possess the formula C<sub>3</sub>H<sub>7</sub>Cl, but the chlorine atom is located at different positions.
-
Functional Group Isomerism: This type involves isomers with different functional groups. For example, ethanol (CH<sub>3</sub>CH<sub>2</sub>OH) and dimethyl ether (CH<sub>3</sub>OCH<sub>3</sub>) are functional group isomers; both have the formula C<sub>2</sub>H<sub>6</sub>O, but one has an alcohol functional group (-OH), while the other possesses an ether functional group (-O-).
2. Stereoisomerism (Spatial Isomerism): Stereoisomers possess the same connectivity but differ in the spatial arrangement of their atoms. This category is further divided into:
-
Geometric Isomerism (cis-trans isomerism): This type of isomerism occurs in molecules with restricted rotation, usually due to the presence of a double bond or a ring structure. 'Cis' isomers have similar groups on the same side of the double bond or ring, while 'trans' isomers have them on opposite sides. For example, cis-2-butene and trans-2-butene are geometric isomers.
-
Optical Isomerism (Enantiomerism): This arises when a molecule is chiral, meaning it is non-superimposable on its mirror image. These mirror images are called enantiomers. A key feature of chiral molecules is the presence of at least one asymmetric carbon atom (a carbon atom bonded to four different groups). Enantiomers have identical physical properties except for their interaction with plane-polarized light. One rotates the light clockwise (dextrorotatory, +), while the other rotates it counterclockwise (levorotatory, -). A mixture of equal amounts of both enantiomers is called a racemic mixture.
-
Diastereoisomerism: These are stereoisomers that are not mirror images of each other. They arise when a molecule has more than one chiral center. Diastereomers differ in their physical and chemical properties.
Identifying Isomers: Techniques and Approaches
Determining the type of isomerism present requires a combination of analytical techniques:
-
Spectroscopy: Techniques like Nuclear Magnetic Resonance (NMR) spectroscopy and Infrared (IR) spectroscopy are crucial in elucidating the structure of molecules. NMR provides information about the connectivity and chemical environment of atoms, while IR spectroscopy identifies functional groups based on their characteristic absorption patterns.
-
Mass Spectrometry (MS): MS helps determine the molecular weight and fragmentation pattern of a molecule, providing clues about its structure.
-
X-ray Crystallography: This technique offers a detailed three-dimensional structure of a molecule, especially useful in confirming the spatial arrangement of atoms in stereoisomers.
-
Boiling Point and Melting Point Determination: These physical properties often differ significantly between isomers, providing initial clues about their structural differences.
Real-World Implications of Isomerism
The existence of isomers has significant implications across various fields:
1. Pharmaceuticals: Isomers can exhibit vastly different pharmacological activities. One isomer might be therapeutically active, while another could be inactive or even toxic. A classic example is thalidomide, where one enantiomer was a sedative, while the other caused severe birth defects. The importance of isolating and identifying specific isomers in drug development is paramount.
2. Flavors and Fragrances: Isomers in the food and fragrance industry can drastically alter the taste, smell, and overall sensory experience. For instance, different isomers of limonene contribute to the distinct scents of oranges and lemons.
3. Polymers: The arrangement of monomers in polymer chains (isomerism at a macromolecular level) significantly impacts the polymer's properties, such as strength, flexibility, and melting point. This is important in material science and engineering for designing polymers with specific characteristics.
4. Biochemistry: Isomerism plays a crucial role in biological systems. Sugars (glucose, fructose, etc.) are isomers, and their specific structures are critical for their function in metabolism. Similarly, amino acids, the building blocks of proteins, exist as isomers, and their chirality impacts protein structure and function.
A Hypothetical Example: Compounds A and B with the Formula C₄H₈O₂
Let's consider a hypothetical scenario where compounds A and B both have the molecular formula C₄H₈O₂. This formula allows for numerous isomers, including:
-
Esters: Ethyl acetate (CH₃COOCH₂CH₃) is one possibility.
-
Carboxylic Acids: Butanoic acid (CH₃CH₂CH₂COOH) is another potential isomer.
-
Hydroxy Ketones: Several isomers with a hydroxyl group and a ketone group are also possible.
-
Cyclic Compounds: Four-membered ring structures incorporating oxygen can also fit the formula.
Distinguishing between these isomers would require employing the techniques described earlier, such as NMR, IR, and MS. NMR would reveal the different chemical environments of protons and carbons in each isomer, while IR would highlight the characteristic absorption bands of the different functional groups present. Mass spectrometry would corroborate the molecular weight and help identify potential fragmentation patterns associated with specific structures.
Conclusion: The Significance of Unraveling Isomerism
The study of isomers is crucial for a deep understanding of chemical systems. While two compounds might share the same molecular formula, their differing structures result in unique properties with extensive applications and implications across diverse scientific disciplines. From drug development to material science, the ability to identify and characterize isomers is indispensable for advancements in various technological and industrial fields. The techniques for isomer identification are constantly evolving, allowing for increasingly precise characterization and utilization of these fascinating molecules. Future research in isomerism promises to unlock even more potential applications and contribute to a deeper understanding of the intricate world of molecules and their interactions. Further research into this area will continually reveal new insights, leading to breakthroughs in various fields. The importance of understanding isomerism extends beyond the immediate application; it is a cornerstone of modern chemistry, impacting our understanding of matter and its behavior.
Latest Posts
Latest Posts
-
The Standard Overhead Rate Is Computed Separately For
Mar 21, 2025
-
Knowledge Drill 9 7 Serum Appearance
Mar 21, 2025
-
Draw The Expected Major Elimination Product And Identify The Mechanism
Mar 21, 2025
-
In Which Case Can Alphaland Consume At Point Z
Mar 21, 2025
-
Which Of The Following Is An Inside Force For Change
Mar 21, 2025
Related Post
Thank you for visiting our website which covers about Two Compounds A And B Have The Formula . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
