Draw The Expected Major Elimination Product And Identify The Mechanism
Holbox
Mar 21, 2025 · 6 min read
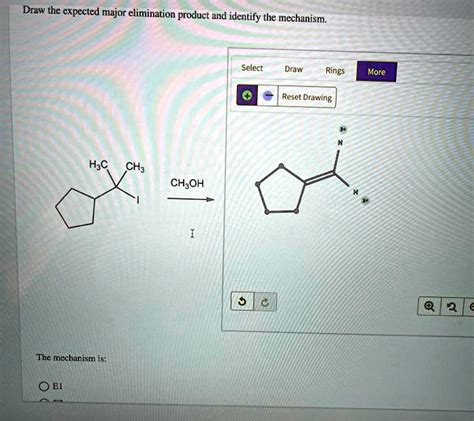
Table of Contents
- Draw The Expected Major Elimination Product And Identify The Mechanism
- Table of Contents
- Drawing the Expected Major Elimination Product and Identifying the Mechanism: A Comprehensive Guide
- Understanding Elimination Reactions: A Foundation
- Key Factors Determining the Major Elimination Product:
- E1 Elimination: A Two-Step Process
- Predicting the Major Product in E1 Reactions:
- E2 Elimination: A Concerted Process
- Predicting the Major Product in E2 Reactions:
- Stereochemistry in Elimination Reactions:
- E2 Stereochemistry:
- E1 Stereochemistry:
- Identifying the Mechanism: Experimental Clues
- Advanced Considerations and Special Cases
- Conclusion
- Latest Posts
- Latest Posts
- Related Post
Drawing the Expected Major Elimination Product and Identifying the Mechanism: A Comprehensive Guide
Elimination reactions are fundamental in organic chemistry, transforming alkyl halides and alcohols into alkenes. Predicting the major product and understanding the underlying mechanism are crucial skills for any organic chemist. This comprehensive guide will delve into the intricacies of elimination reactions, focusing on identifying the major product and elucidating the mechanism involved. We’ll explore various factors influencing product formation and provide numerous examples to solidify your understanding.
Understanding Elimination Reactions: A Foundation
Elimination reactions, broadly categorized as E1 and E2, involve the removal of two substituents from a molecule, typically a hydrogen and a leaving group, to form a π bond. The reaction conditions (strong vs. weak base, protic vs. aprotic solvent, temperature) significantly influence the predominant mechanism and the resulting alkene product.
Key Factors Determining the Major Elimination Product:
Several factors dictate the outcome of an elimination reaction:
-
Substrate Structure: The structure of the alkyl halide or alcohol heavily influences the regioselectivity (which alkene is formed) and stereoselectivity (the stereochemistry of the alkene). Branched substrates often lead to more substituted alkenes (Zaitsev’s rule).
-
Base Strength and Steric Hindrance: Strong, bulky bases favor less substituted alkenes (Hofmann products), while strong, less hindered bases prefer more substituted alkenes (Zaitsev products).
-
Leaving Group: Good leaving groups (e.g., halides, tosylates) facilitate elimination. Poor leaving groups require harsher conditions.
-
Solvent: Protic solvents can stabilize developing charges, often favoring E1 reactions. Aprotic solvents generally favor E2 reactions.
-
Temperature: Higher temperatures often favor elimination over substitution.
E1 Elimination: A Two-Step Process
The E1 mechanism, a unimolecular elimination, proceeds in two steps:
-
Ionization: The leaving group departs, forming a carbocation intermediate. This step is the rate-determining step (RDS), hence the "unimolecular" designation.
-
Deprotonation: A base abstracts a proton from a carbon adjacent to the carbocation, forming a double bond.
Predicting the Major Product in E1 Reactions:
In E1 reactions, the major product is generally the most substituted alkene (Zaitsev's product) due to the greater stability of the more substituted carbocation intermediate. However, carbocation rearrangements can occur, leading to unexpected products. The carbocation can undergo hydride or alkyl shifts to achieve greater stability before deprotonation.
Example: The E1 elimination of 2-bromo-2-methylbutane will primarily yield 2-methyl-2-butene (the more substituted alkene), despite the possibility of forming 2-methyl-1-butene.
(CH3)3CBr → (CH3)3C⁺ + Br⁻ → (CH3)2C=CHCH3 (major) + (CH3)2CHCH=CH2 (minor)
E2 Elimination: A Concerted Process
The E2 mechanism, a bimolecular elimination, is a concerted process, meaning that bond breaking and bond formation occur simultaneously in a single step. It involves a strong base abstracting a proton from a carbon adjacent to the carbon bearing the leaving group.
Predicting the Major Product in E2 Reactions:
Predicting the major product in E2 reactions depends on both the base and the substrate.
-
Zaitsev's Rule: With strong, less hindered bases, the major product is usually the more substituted alkene (Zaitsev product), due to the greater stability of the alkene.
-
Hofmann's Rule: With strong, bulky bases, the major product can be the less substituted alkene (Hofmann product) due to steric hindrance. The bulky base preferentially abstracts a proton from the less hindered position, minimizing steric interactions.
Example: The E2 elimination of 2-bromobutane with potassium tert-butoxide (a bulky base) will predominantly yield 1-butene (Hofmann product), while the reaction with a less hindered base like sodium ethoxide would yield 2-butene (Zaitsev product).
2-bromobutane + potassium tert-butoxide → CH3CH2CH=CH2 (major, Hofmann) + CH3CH=CHCH3 (minor, Zaitsev)
2-bromobutane + sodium ethoxide → CH3CH=CHCH3 (major, Zaitsev) + CH3CH2CH=CH2 (minor, Hofmann)
Stereochemistry in Elimination Reactions:
The stereochemistry of both the substrate and the product is crucial in elimination reactions.
E2 Stereochemistry:
E2 reactions generally proceed via an anti-periplanar geometry. This means that the hydrogen being abstracted and the leaving group must be on opposite sides of the molecule (diaxial in cyclohexanes). This anti-coplanar arrangement allows for optimal orbital overlap during the concerted reaction. This requirement often dictates the stereochemistry of the product alkene. If the substrate is chiral, the stereochemistry of the starting material affects the stereochemistry of the alkene formed, yielding either a cis or trans isomer.
E1 Stereochemistry:
E1 reactions are less stereospecific than E2 reactions. The formation of the planar carbocation intermediate allows for rotation about the C-C bonds before deprotonation. This results in a mixture of cis and trans alkenes, often favoring the more stable trans isomer.
Identifying the Mechanism: Experimental Clues
Determining whether an elimination reaction proceeds via an E1 or E2 mechanism requires careful consideration of the experimental conditions and product distribution.
-
Rate Dependence: E1 reactions are first-order (rate depends only on the concentration of the substrate), while E2 reactions are second-order (rate depends on the concentration of both the substrate and the base).
-
Substrate Structure: Highly branched substrates often favor E1, while less branched substrates can undergo both E1 and E2.
-
Base Strength: Strong bases favor E2, while weak bases or no added base favor E1.
-
Solvent: Protic solvents often promote E1, whereas aprotic solvents promote E2.
-
Product Distribution: The ratio of Zaitsev to Hofmann products can provide clues about the base's steric hindrance and the mechanism.
By analyzing these factors, one can confidently predict the mechanism and the major elimination product.
Advanced Considerations and Special Cases
-
Competition between Elimination and Substitution: Often, both elimination and substitution reactions can occur simultaneously, especially with secondary and tertiary substrates. The relative rates of these competing reactions are influenced by the factors discussed above.
-
Allylic and Benzylic Eliminations: These eliminations often occur readily due to the resonance stabilization of the resulting allylic or benzylic carbocations or carbanions.
-
Pyrolytic Elimination: This type of elimination involves heating an ester, commonly a xanthate ester, to eliminate a thiocarboxylic acid and produce an alkene. This reaction is stereospecific, favoring the formation of the less substituted alkene (Hofmann product).
Conclusion
Predicting the major product and determining the mechanism of an elimination reaction is a critical skill for organic chemists. A thorough understanding of factors such as substrate structure, base strength, steric hindrance, solvent effects, and temperature, along with an appreciation of the stereochemical implications of E1 and E2 mechanisms, is essential for successfully navigating these reactions. This comprehensive guide provides a strong foundation for tackling diverse elimination reaction problems, enabling you to confidently draw the expected major product and identify the prevailing mechanism. Continued practice with a wide array of examples will further hone your proficiency in this crucial area of organic chemistry.
Latest Posts
Latest Posts
-
Suppose Your Expectations Regarding The Stock Market Are As Follows
Mar 28, 2025
-
Which Example Best Illustrates Hindsight Bias
Mar 28, 2025
-
What Are Good Recovery Measures To Incorporate In Your Organization
Mar 28, 2025
-
Lily Works In A Hospital Human Resources Department
Mar 28, 2025
-
At What Point Are The Atria Repolarizing
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about Draw The Expected Major Elimination Product And Identify The Mechanism . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
