The Reaction Of A Certain Alcohol With Hbr
Holbox
Mar 27, 2025 · 6 min read
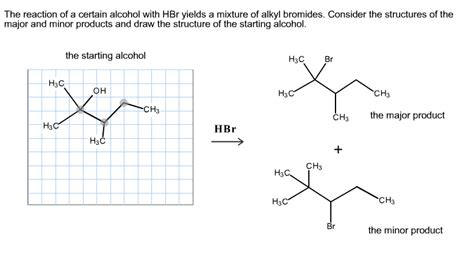
Table of Contents
- The Reaction Of A Certain Alcohol With Hbr
- Table of Contents
- The Reaction of Alcohols with HBr: A Comprehensive Overview
- The Mechanism of Alcohol-HBr Reaction
- SN1 Mechanism: Favored by Tertiary and Some Secondary Alcohols
- SN2 Mechanism: Favored by Primary Alcohols
- Factors influencing the reaction mechanism and rate
- Regioselectivity and Stereochemistry
- Regioselectivity in SN1 Reactions
- Stereochemistry in SN1 and SN2 Reactions
- Synthetic Applications
- Practical Considerations and Safety
- Comparison with Other Methods of Alkyl Halide Synthesis
- Conclusion
- Latest Posts
- Latest Posts
- Related Post
The Reaction of Alcohols with HBr: A Comprehensive Overview
The reaction of alcohols with hydrogen bromide (HBr) is a fundamental organic chemistry reaction, resulting in the formation of alkyl halides. This transformation is crucial in organic synthesis, providing a versatile pathway to introduce a halogen atom onto a carbon skeleton. Understanding the mechanism, factors influencing the reaction, and applications of this transformation is essential for any aspiring organic chemist. This comprehensive article will delve into the intricacies of this reaction, exploring its mechanism, regioselectivity, stereochemistry, and synthetic applications.
The Mechanism of Alcohol-HBr Reaction
The reaction between an alcohol and HBr proceeds via an SN1 or SN2 mechanism, depending on the structure of the alcohol and the reaction conditions. The choice of mechanism significantly impacts the stereochemical outcome and the overall rate of the reaction.
SN1 Mechanism: Favored by Tertiary and Some Secondary Alcohols
Tertiary alcohols generally react with HBr via an SN1 mechanism. This is a two-step process:
-
Protonation of the hydroxyl group: The oxygen atom of the alcohol is protonated by HBr, converting the poor leaving group –OH into a much better leaving group, water (H₂O). This step is rapid and reversible.
R₃C-OH + HBr ⇌ R₃C-OH₂⁺ + Br⁻ -
Heterolytic Cleavage and Nucleophilic Attack: The protonated alcohol undergoes heterolytic cleavage, forming a carbocation intermediate. The bromide ion (Br⁻) then acts as a nucleophile, attacking the carbocation to form the alkyl halide.
R₃C-OH₂⁺ → R₃C⁺ + H₂O R₃C⁺ + Br⁻ → R₃C-Br
The formation of a stable carbocation is the rate-limiting step in the SN1 mechanism. Therefore, tertiary alcohols, which form relatively stable tertiary carbocations, react readily via SN1. Secondary alcohols can also react via SN1, but the reaction rate is significantly slower due to the less stable secondary carbocation.
SN2 Mechanism: Favored by Primary Alcohols
Primary alcohols typically react with HBr via an SN2 mechanism. This is a concerted, one-step process where the nucleophile (Br⁻) attacks the carbon atom bearing the hydroxyl group from the backside, simultaneously with the departure of the leaving group (water).
R-CH₂-OH + HBr → R-CH₂-Br + H₂O
The SN2 mechanism is stereospecific, resulting in inversion of configuration at the carbon atom undergoing substitution. This means that if the starting alcohol is chiral, the resulting alkyl halide will have the opposite configuration.
Factors influencing the reaction mechanism and rate
Several factors influence the reaction mechanism and its rate:
-
Structure of the alcohol: As mentioned above, tertiary alcohols favor SN1, primary alcohols favor SN2, and secondary alcohols can react through either mechanism depending on the specific conditions.
-
Concentration of HBr: Higher concentrations of HBr favor SN1 mechanisms by increasing the protonation rate of the alcohol and providing a higher concentration of bromide ions for nucleophilic attack.
-
Temperature: Higher temperatures generally increase the reaction rate, favoring SN1 mechanisms.
-
Solvent: The solvent can influence the reaction rate and mechanism. Polar protic solvents generally favor SN1 reactions, while polar aprotic solvents can favor SN2 reactions.
Regioselectivity and Stereochemistry
Regioselectivity in SN1 Reactions
In SN1 reactions involving secondary and tertiary alcohols, the formation of the carbocation intermediate leads to the possibility of regioselectivity if the carbon atom bearing the hydroxyl group is substituted. More substituted carbocations are more stable (due to hyperconjugation and inductive effects), resulting in the major product being formed from the more substituted carbocation. This is governed by Markovnikov's rule, which states that the hydrogen atom adds to the carbon atom with the greater number of hydrogen atoms.
Stereochemistry in SN1 and SN2 Reactions
SN1 reactions lead to racemization at the chiral center if the reaction involves a secondary or tertiary alcohol. The planar carbocation intermediate can be attacked from either side by the nucleophile, resulting in a mixture of enantiomers.
SN2 reactions, on the other hand, are stereospecific, resulting in inversion of configuration at the carbon atom undergoing substitution. This means if the starting alcohol is chiral, the product will have the opposite configuration.
Synthetic Applications
The reaction of alcohols with HBr is a valuable tool in organic synthesis, enabling the preparation of a wide range of alkyl halides. Alkyl halides are important intermediates in numerous organic transformations, including:
-
Grignard reagent synthesis: Alkyl halides can be converted into Grignard reagents, which are versatile nucleophiles used in a variety of carbon-carbon bond-forming reactions.
-
Williamson ether synthesis: Alkyl halides react with alkoxides to form ethers.
-
Substitution reactions: Alkyl halides can participate in various substitution reactions, enabling the introduction of various functional groups.
-
Elimination reactions: Alkyl halides can undergo elimination reactions to form alkenes.
-
Preparation of other functional groups: Alkyl halides serve as precursors to numerous functional groups like alcohols, amines, nitriles and carboxylic acids.
Practical Considerations and Safety
The reaction of alcohols with HBr often requires anhydrous conditions and is typically carried out in the presence of a strong acid catalyst. The reaction can be exothermic, so careful control of the reaction temperature is crucial. HBr is a corrosive and toxic gas, so appropriate safety precautions must be taken when handling this reagent.
Comparison with Other Methods of Alkyl Halide Synthesis
While the reaction of alcohols with HBr is a common method for synthesizing alkyl halides, other methods also exist, each with its own advantages and disadvantages:
-
Reaction with HX (HCl, HI): Similar to HBr, other hydrogen halides (HCl and HI) can also react with alcohols to produce alkyl halides. However, the reactivity varies greatly, with HI being more reactive than HBr, and HCl being the least reactive.
-
Appel reaction: The Appel reaction uses triphenylphosphine and carbon tetrachloride or other halocarbons to convert alcohols into alkyl halides. This method is suitable for alcohols that may not react well with HX.
-
Substitution using SOCl₂ or PBr₃: Thionyl chloride (SOCl₂) and phosphorus tribromide (PBr₃) are used to convert alcohols into alkyl chlorides and bromides, respectively. These reagents offer advantages in terms of selectivity and ease of handling compared to direct reaction with gaseous HBr.
Conclusion
The reaction of alcohols with HBr offers a straightforward and effective method for the synthesis of alkyl halides. Understanding the reaction mechanism, regioselectivity, stereochemistry, and influencing factors allows for the rational design of synthetic routes and the prediction of product outcomes. The widespread applications of alkyl halides in organic chemistry solidify the importance of this fundamental transformation, making it an essential topic for all organic chemistry students and researchers. The choice of method, however, should always be guided by the specific alcohol structure, desired product, and the availability of reagents, highlighting the versatility and importance of understanding the intricacies of this reaction. Careful planning and consideration of safety protocols are paramount when conducting this reaction.
Latest Posts
Latest Posts
-
Which Of The Following Events Would Increase Producer Surplus
Mar 31, 2025
-
Assesses The Consistency Of Observations By Different Observers
Mar 31, 2025
-
A Business Plan Is A Document That Outlines Blank
Mar 31, 2025
-
The Theory We Have Constructed Originates With The Three Phases
Mar 31, 2025
-
Which Was Not True About Egyptian Views Of The Afterlife
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about The Reaction Of A Certain Alcohol With Hbr . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
